Construction and using method of pichia pastoris expression vector facilitating achievement of natural N end expression of protein
A construction method and vector technology, applied in the construction field of Pichia pastoris expression vector, can solve the problems such as complicated construction process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
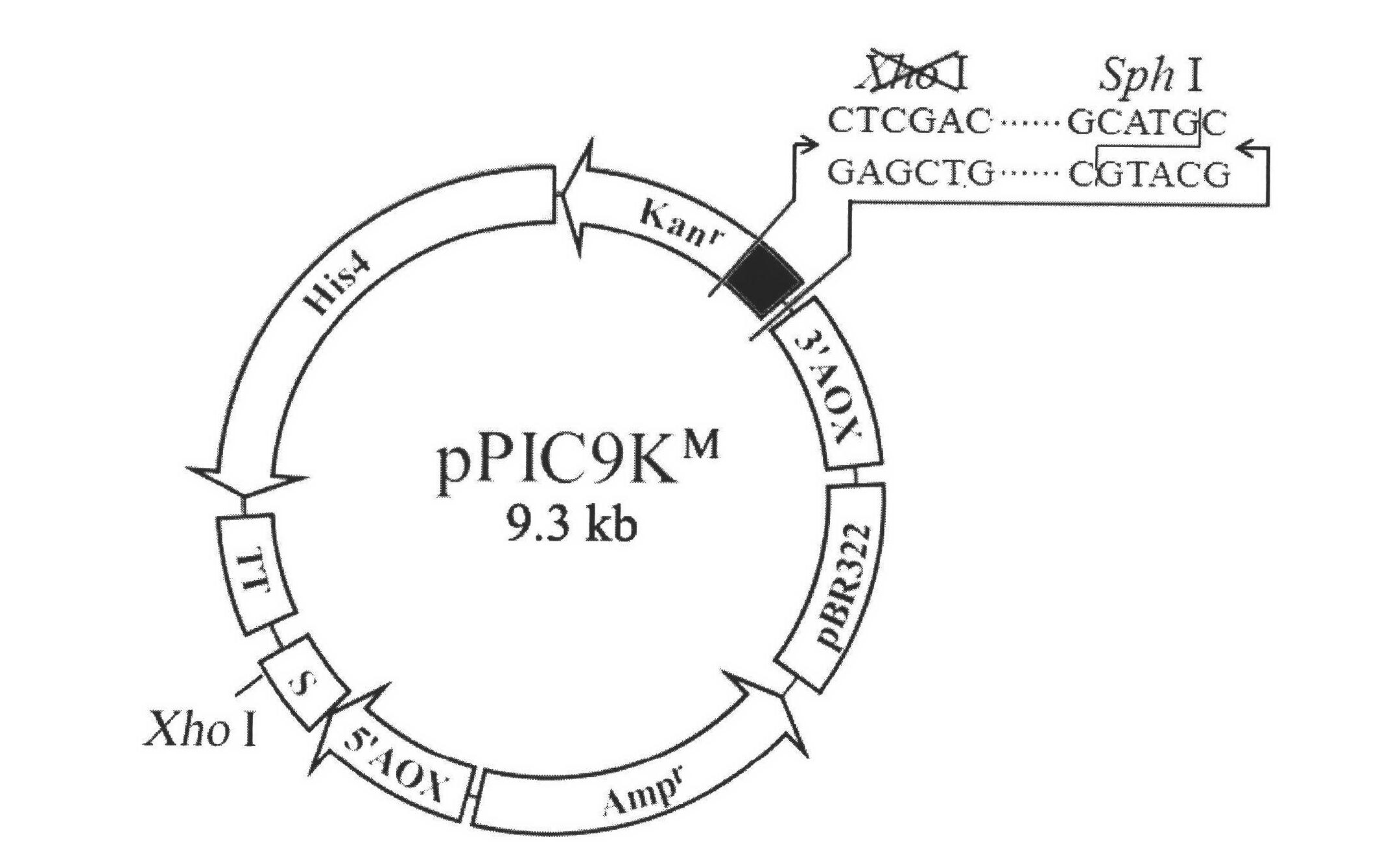
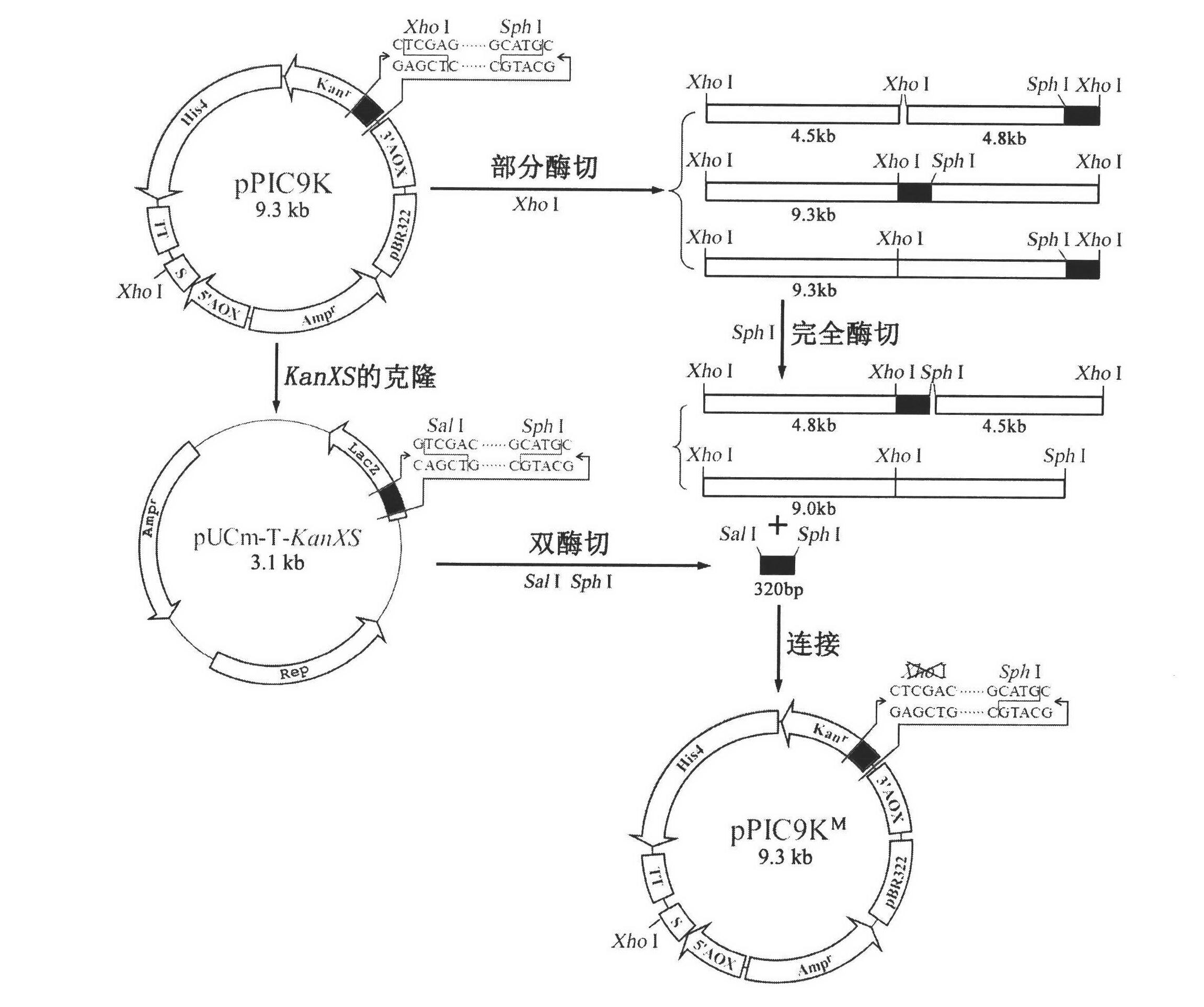
[0032] Example 1 Plasmid pPIC9K M build
[0033] Plasmid pPIC9K M The build process is as follows figure 2 ,Specific steps are as follows:
[0034] 1) Design a pair of forward and reverse primers KanF and KanR according to the sequence between Xho I and Sph I at the Kanna resistance gene in pPIC9K,
[0035] KanF: 5′- GTC GAC CAAGACGTTTCCC-3' (SEQ ID NO. 1);
[0036] KanR: 5′- GTGCAT GCAAGGAGATGGC-3' (SEQ ID NO. 2);
[0037] The 5' end of KanF contains a Sal I restriction site (shown underlined), and the 5' end of KanR contains a Sph I restriction site (shown underlined). Under the action of KanF and KanR, the KanXS fragment was amplified by PCR using the pPIC9K plasmid as a template (94°C for 3min; 30 cycles, 94°C for 30s, 55°C for 30s, 72°C for 30s; 72°C for 10min). The PCR product was analyzed by 0.7% agarose gel electrophoresis, the target band was recovered and ligated with pUCm-T (pUCm-T-KanXS), transformed into Escherichia coli JM109, and sent to Shanghai Sang...
Embodiment 2
[0039] Example 2 Construction and heterologous expression of the natural N-terminal expression plasmid of the 10th family xylanase of Aspergillus usami
[0040]1) Synthetic primer XynCF2: 5'- CTCGAG AAAAGACAGGCTTCAGTGAGTATTGA-3', XynCR2:5'- GCGGCCGC CTAGAGAGCATTTGCGATAG-3′, underlined are Xho I and Not I restriction sites respectively. Using the vector pUCm-T-Ausxyn10A plasmid as a template, the xylanase gene Ausxyn10A was obtained by PCR (94°C for 3min; 30 cycles, 94°C for 30s, 56°C for 30s, 72°C for 1min; 72°C for 10min). After agarose gel electrophoresis analysis, the target band was recovered and ligated with pUCm-T (pUCm-T-Ausxyn10A'), transformed into Escherichia coli JM109, and sent to Shanghai Sangon for sequencing after being identified by enzyme digestion.
[0041] 2) Combine the sequenced correct pUCm-T-Ausxyn10A' with pPIC9K M Plasmids were double digested with Xho I and Not I, and the recovered digested products were ligated under the action of T4DNA ligase t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com