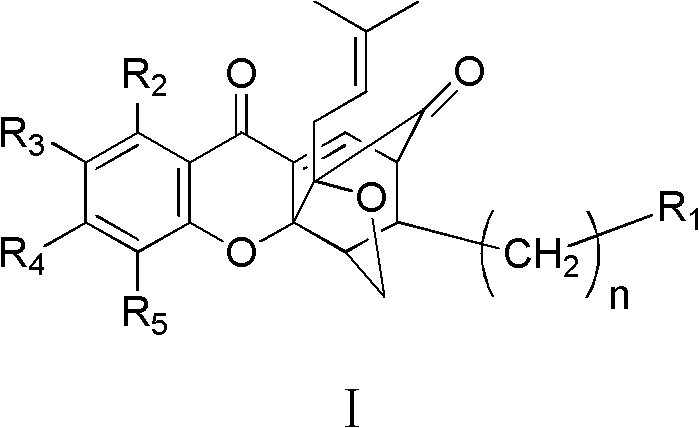
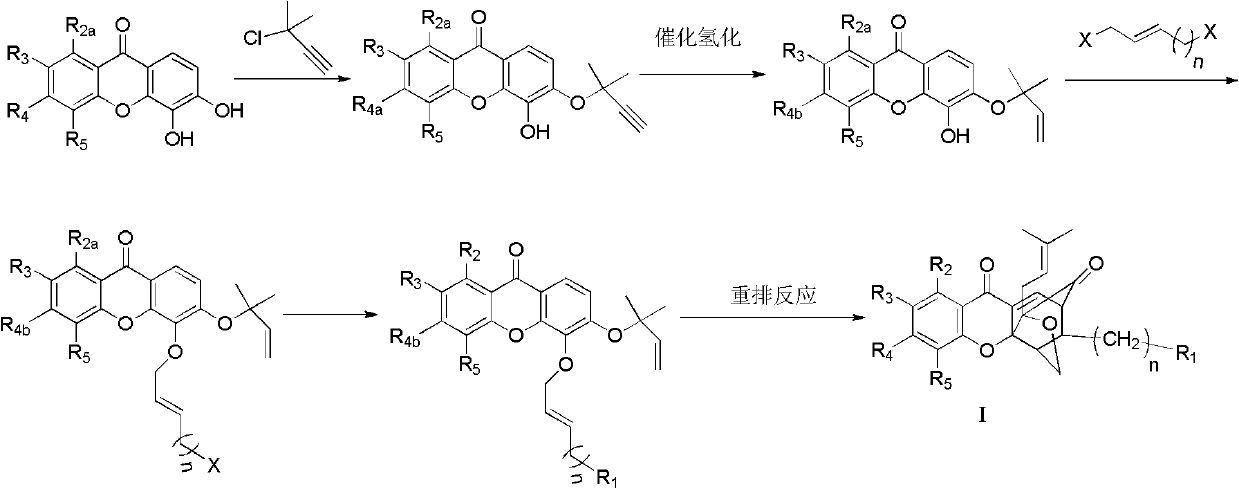
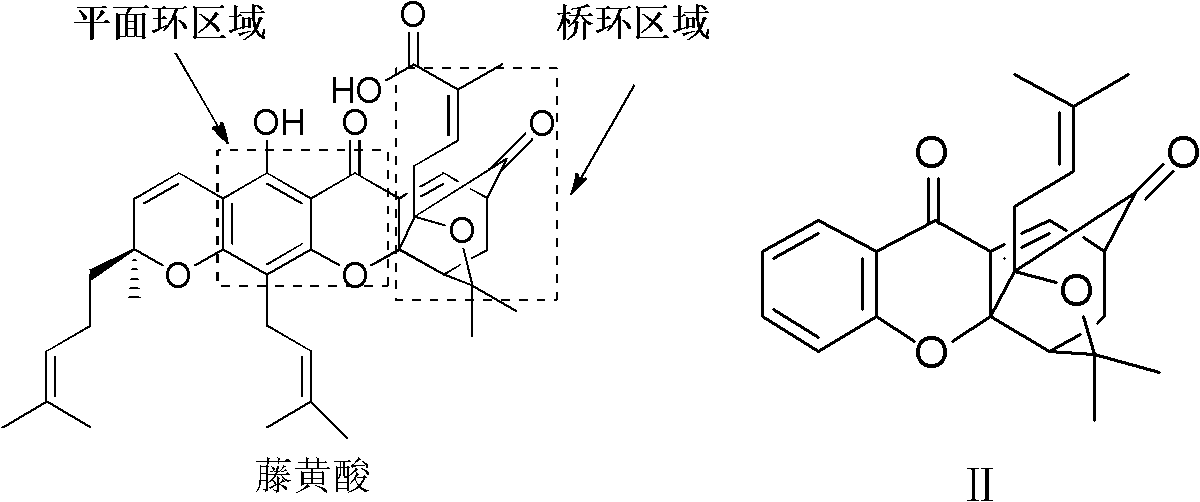
Garcinia derivative and preparation method and medicinal application thereof
A pharmacy and compound technology, applied in the field of Garcinia derivatives and their preparation, can solve the problems of limited structure modification space, lack of druggability characteristics, etc., and achieve the effect of improving gastrointestinal absorption, novel structure and excellent activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] 3,3a,4,5-tetrahydro-1,11-bis(3-methylbut-2-en-1-yl)-4-bromomethyl-8,10-dihydroxy-1,5-ylidene Methylbridge-1H, 7H-furo[3,4-d]xanthene-7,13-dione (CPUYZ001)
[0054]
[0055] (1) Preparation of 1,5-dihydroxy-3,6-bis[(2-methylbut-3-yn-2-yl)oxy]-9H-xanthene-9-one
[0056] Dissolve 1,3,5,6-tetrahydroxy-9H-xanthene-9-one (5.2g, 20mmol) in acetone (100mL), add potassium carbonate (8.28g, 60mmol), potassium iodide (9.96g, 60mmol ), ketone iodide (382mg, 2mmol) and 3-chloro-3-methylbut-1-yne (6.7mL, 60mmol), heated to 60°C for 1h. After filtration, the filtrate was concentrated, and the residue was subjected to column chromatography (petroleum ether: ethyl acetate = 8:1) to obtain 1.19 g of a yellow solid, yield 15.2%, m.p.149-150°C. 1 H NMR (300MHz, CDCl 3 ): δ1.75(s, 6H, 2×-C H 3 ), 1.79(s, 6H, 2×-C H 3 ), 2.71(d, 2H, 2×-C≡C H ), 5.84(s, 1H, Ar-O H ), 6.66 (d, J=2.4Hz, 1H, Ar- H ), 6.91 (d, J=2.4Hz, 1H, Ar- H ), 7.54 (d, J=9.0Hz, 1H, Ar- H ), 7.76 (d, J=9.0Hz, 1...
Embodiment 2
[0064] 3,3a,4,5-tetrahydro-1,11-bis(3-methylbut-2-en-1-yl)-4-iodomethyl-8,10-dihydroxy-1,5-ylidene Methylbridge-1H, 7H-furo[3,4-d]xanthene-7,13-dione (CPUYZ002)
[0065]
[0066] (1) (E)-1-hydroxy-5-((4-iodobut-2-en-1-yl)oxy)-3,6-bis[(2-methylbut-3-ene-2 Preparation of -yl)oxyl]-9H-xanthene-9-one
[0067] (E)-1-hydroxyl-5-((4-bromobut-2-en-1-yl)oxy)-3,6-bis[(2-methylbut-3-en-2-yl )Oxy]-9H-xanthene-9-one (200mg, 0.378mmol) was dissolved in acetone (10mL), sodium iodide (114mg, 0.756mmol) was added, and reacted at room temperature. After TLC detection, the reaction solution was filtered after the raw materials disappeared, the filtrate was concentrated, and the residue was chromatographed (petroleum ether: ethyl acetate = 8:1) to obtain 191 mg of a yellow oil, with a yield of 87.2%. 1 HNMR (300MHz, CDCl 3 ): δ1.51(s, 12H, CH 3 ×4), 3.79(d, 2H, CH 2 ), 4.56 (d, 2H, CH 2 ), 5.05~5.35(m, 4H, CH=C H 2 ×2), 5.95~6.15(m, 4H, C H =CH 2 ×2, C H =CH×2), 6.36(d, J=2.1Hz, 1H...
Embodiment 3
[0071] 3,3a,4,5-tetrahydro-1,11-di(3-methylbut-2-en-1-yl)-4-thiocyanatomethyl-8,10-dihydroxy-1,5 -Methylenebridge-1H, 7H-furo[3,4-d]xanthene-7,13-dione (CPUYZ003)
[0072]
[0073] Prepared according to the method of Example 2, using potassium thiocyanide instead of sodium iodide, and 95% ethanol instead of acetone as solvent to obtain 60 mg of yellow oil, with a two-step yield of 30.4%. IR (KBr, cm -1 ): 2968, 2908, 2155, 1739, 1638, 1595, 1431, 1371, 1223, 1134, 826; 1 H NMR (300MHz, CDCl 3 ): δ1.10~1.81(m, 12H), 2.35~2.75(m, 4H), 2.90(m, 1H), 3.25(m, 2H), 3.65(d, 2H), 3.92(m, 1H), 4.29(m, 1H), 4.47(m, 1H), 5.18(m, 1H), 6.02(s, 1H), 7.07(d, J=6.9Hz, 1H), 12.30(s, 1H); EI-MS (m / z)507[M] + , 479, 448, 396
[0074] ESI-HRMS(m / z)calcd for C 28 h 29 NO 6 S[M+H] + , 508.1716, Found, 508.1768.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com