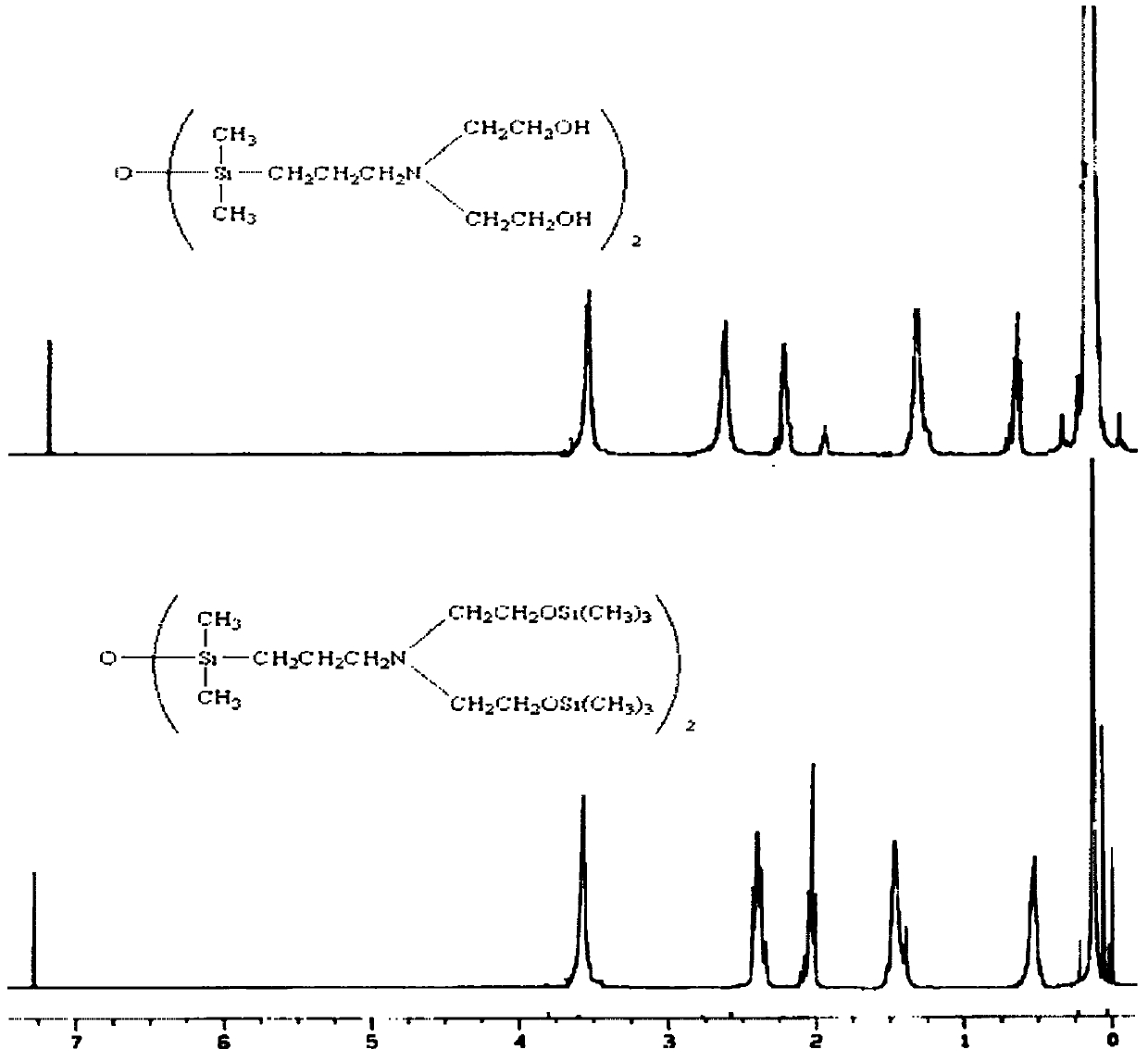
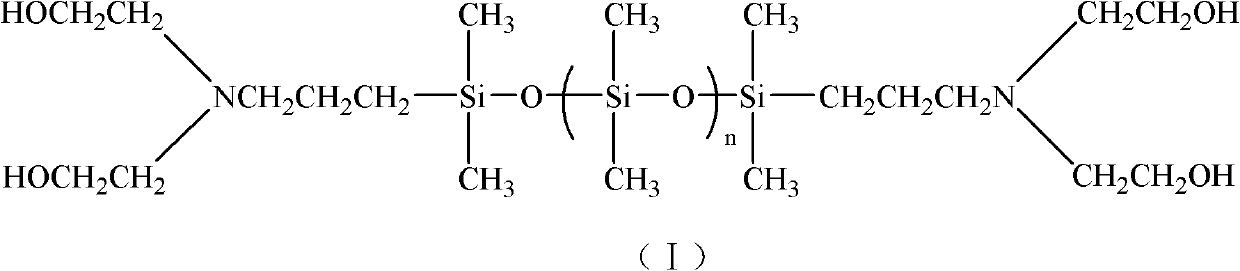
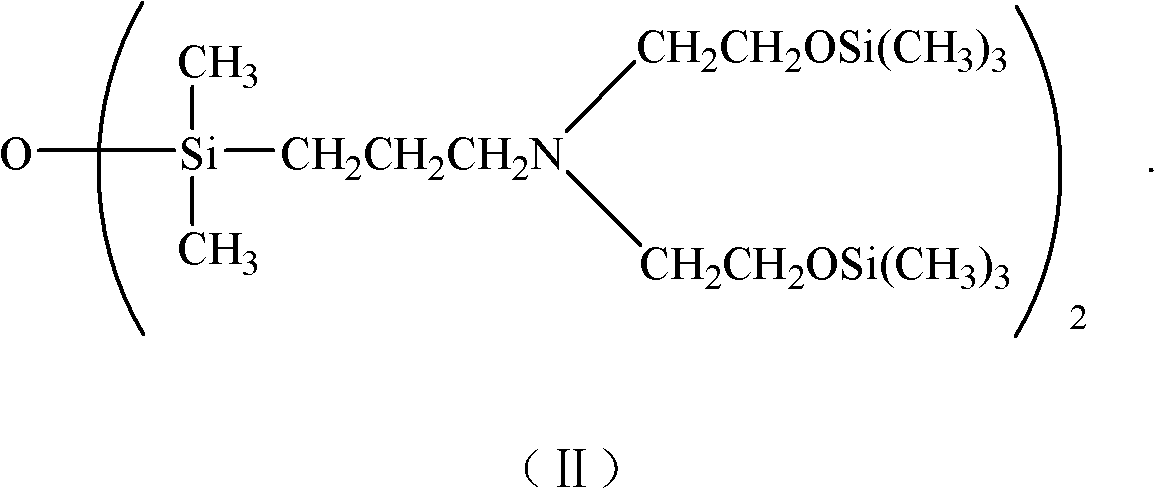
Alpha,omega-(dihydroxyethyl) aminopropyl terminated polysiloxane, synthetic method and midbody thereof
A synthesis method and technology of polysiloxane, applied in the field of α, can solve the problems of unsaturated compounds being difficult to obtain, unsaturated compound reaction conditions are harsh, etc., and achieve the advantages of industrial application, easy availability of raw materials, high product yield and purity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Hydroxyl protection reaction (molar ratio diethanolamine: hexamethyldisilazane=1:1.5)
[0028] In a 250mL three-necked flask, add 52.50g of diethanolamine. At room temperature, slowly add 12110g of hexamethyldisilazane dropwise. reaction. The reaction solution was distilled under atmospheric pressure, and the fraction at 218-220° C. was collected to obtain 120.70 g of bis(trimethylsiloxy)ethylamine, with a yield of 97%. The compound corresponds to 1 H NMR (CDCl 3 , ppm) analysis data are as follows: 0.03 ~ 0.22 (m, 18H, Si-C H 3 ), 1.83(s, 1H, N H ), 2.73~2.75(t, 4H, N-C H 2 ), 3.69~3.71(t, 4H, O-C H 2 ).
[0029] Alkylation reaction (molar ratio: allyl bromide: bis(trimethylsilyloxy) ethylamine: triethylamine=1.2:1:1.5)
[0030]In a 250 mL three-necked flask, 99.60 g of bis(trimethylsiloxy)ethylamine, 60.60 g of triethylamine and 100 mL of dichloromethane were sequentially added. Place the three-neck flask in an ice-water bath, and slowly add 58.08 g of all...
Embodiment 2
[0042] Change the ratio of the amount of diethanolamine and hexamethyldisilazane in the hydroxyl protection reaction of Example 1 to 1:1.2, the reaction temperature is changed to 110°C, the reaction time is changed to 6h, and other reaction conditions are as in Example 1 As described, bis(trimethylsiloxy)ethylamine was obtained with a yield of 93%.
Embodiment 3
[0044] Allyl bromide is changed to allyl chloride in the alkylation reaction of embodiment 1, and the ratio of the amount of substances of allyl chloride, two (trimethylsilyloxy) ethylamine and triethylamine becomes 2 : 1: 3, other reaction conditions are as described in Example 1, N, N-bis(trimethylsilyloxy) ethylallylamine, the yield is 82%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Tensile strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com