High-activity probiotic composition suitable for infants and preparation method thereof
A technology for probiotics and infants, which is applied in the field of highly active probiotic compositions and their preparation, can solve the problems of translocation of flora, higher requirements for the use of probiotics, damage to the growth conditions of beneficial bacteria, etc., and meets the requirements of temperature and humidity Strict, gut health-boosting, immune-boosting effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
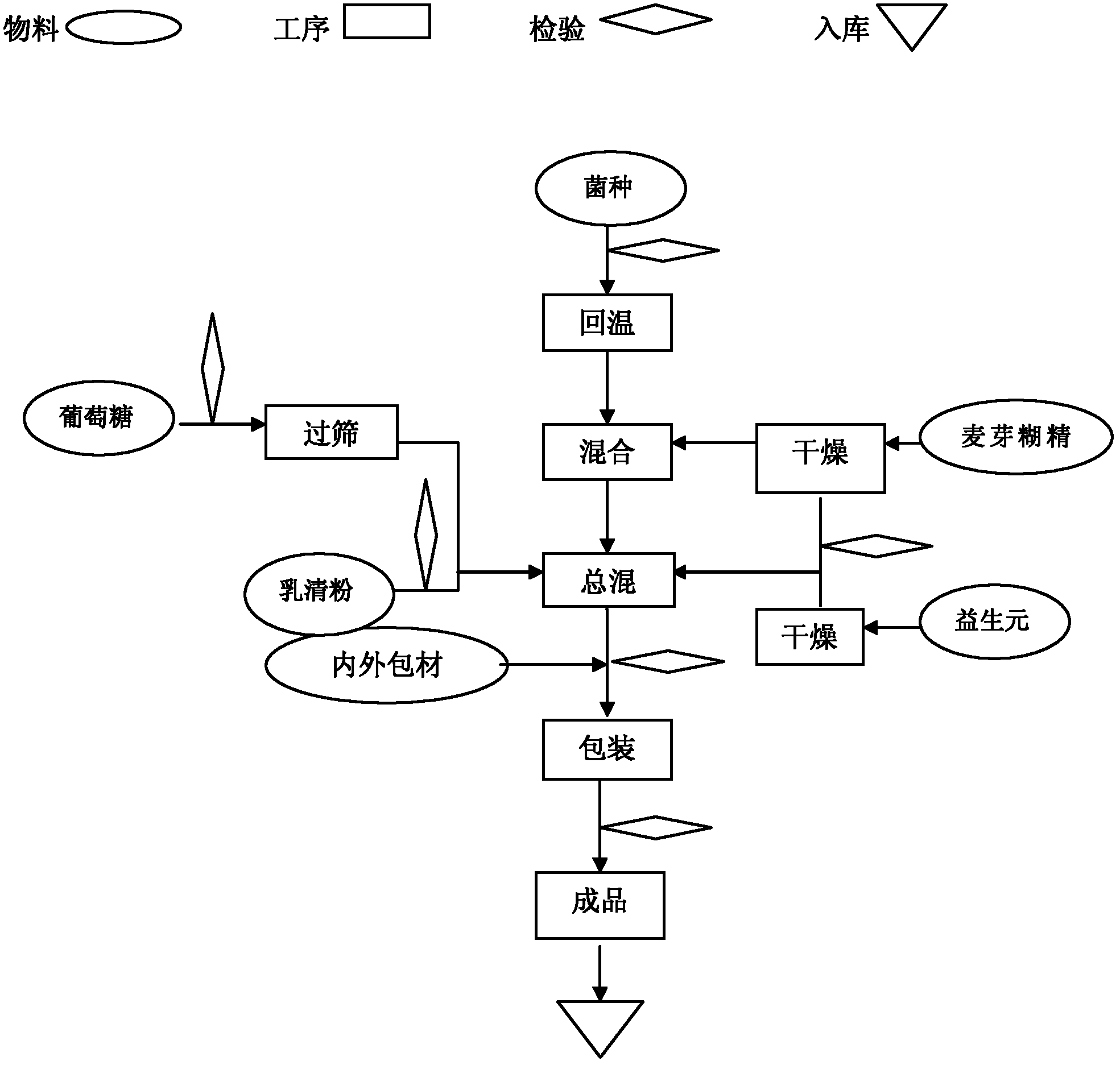
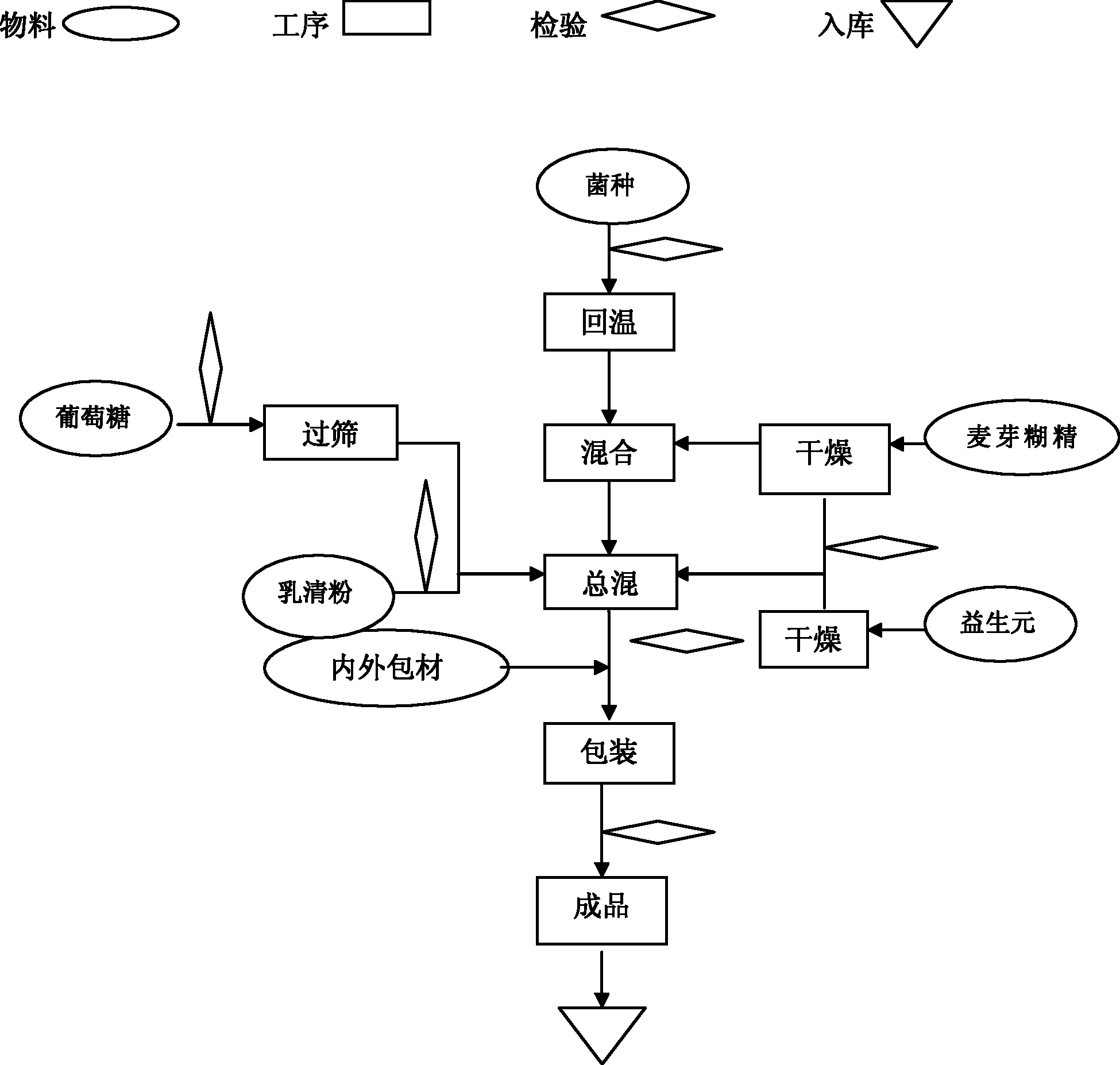
[0046] Such as figure 1 Shown preparation process, preparation method of the present invention is:
[0047] ① All the excipients need to be dried until the water content is ≤1.5%, and the water activity (Aw) is ≤0.1;
[0048] ② After the probiotics are taken out from the cold storage, return to the temperature at low temperature (≤18°C) for 10 hours;
[0049] ③The temperature in the mixing room is controlled at ≤18°C, and the relative humidity (RH) is ≤35%;
[0050] ④ Replace the air in the mixer with dry medical nitrogen;
[0051] ⑤ Add a small amount of maltodextrin (5 times the amount of probiotics), then add probiotics, mix for 5 minutes, then add the remaining maltodextrin and the remaining raw and auxiliary materials, mix for 20 minutes, and wait for discharge;
[0052] ⑥ Replace the air in the container with dry nitrogen and fill it, and store it in a sealed container, or pack it in vacuum.
[0053]⑦ During the packing process, fill the hopper with nitrogen gas, and...
Embodiment 1
[0055] Embodiment 1: solid beverage
[0056] Bifidobacterium lactis BI-071kg, Bifidobacterium lactis HN019 1.3kg, Bifidobacterium animalis Bb-12 1kg, maltodextrin 60kg, glucose 20kg, whey powder 11.7kg, galacto-oligosaccharide 5kg.
[0057] The preparation method and process requirements, the process is as follows figure 1 Shown:
[0058] The first step: raw material pretreatment
[0059] 1. Lactobacillus acidophilus and Bifidobacterium lactis need to be stored in the freezer and warmed up at 15±5°C for 10 hours before production;
[0060] 2. Raw materials with larger particles need to pass through a 40-mesh sieve;
[0061] 3. Raw materials with high water activity need to be dried to make the water activity (Aw) ≤ 0.1;
[0062] Step Two: Production Requirements
[0063] 1. Workshop requirements: temperature ≤ 18 ℃, humidity ≤ 35%;
[0064] Step Three: Mixing
[0065] First replace the air in the mixer with dry medical nitrogen, put in a small amount of pretreated malto...
Embodiment 2
[0071] Embodiment 2: tablet
[0072] Bifidobacterium lactis BI-07 0.5kg, Bifidobacterium animalis Bb-12 0.5kg, Lactobacillus acidophilus NCFM 0.5kg, maltodextrin 45kg, glucose 20kg, whey powder 20kg, fructooligosaccharide 10kg, magnesium stearate 3.5kg, appropriate amount of adhesive.
[0073] Preparation method and process requirements:
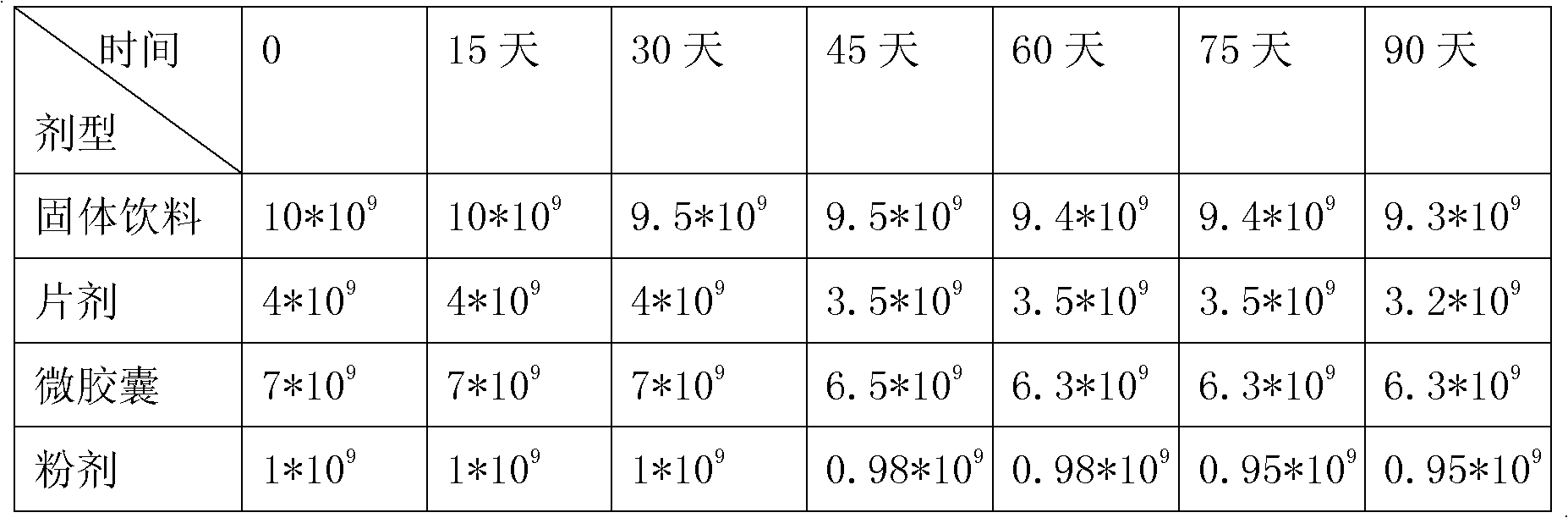
[0074] Maltodextrin, glucose, whey powder, and fructooligosaccharides were mixed, granulated and dried to a water activity (Aw)≤0.1, mixed according to the mixing method in Example 1, and tableted. Made with probiotics 4*10 9 cfu / g tablet, the daily dosage for infants aged 1-3 is 1-2g.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com