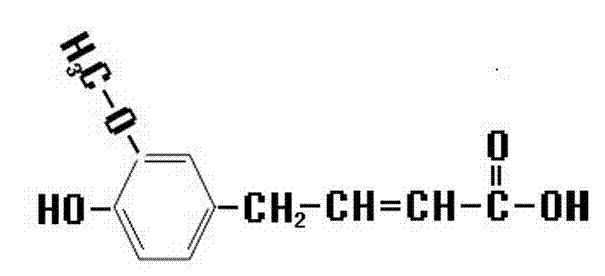
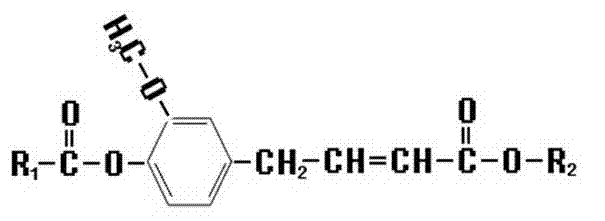
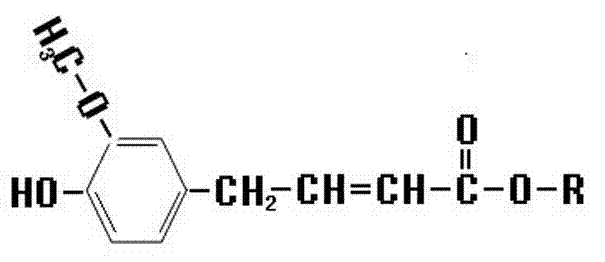
Synthetic method of novel ferulic acid derivative
A synthesis method and a technology of derivatives, which are applied in the field of medicine, can solve problems such as inability to solve stability problems, affect the therapeutic effect of products, and attenuate anti-oxidation ability, and achieve strong blood vessel expansion function, strong anti-oxidation ability, and strong anticoagulant effect Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0028] Implementation example one:
[0029] Synthetic method of ethyl acetyl ferulate
[0030] Put 10~20g of ethyl ferulate and 30~100ml of tetrahydrofuran in a reaction vessel, stir, add 8~20g of anhydrous sodium carbonate after completely dissolving, and weigh 5~15g of acetic anhydride in the funnel, add slowly in a normal temperature water bath Acetic anhydride, stirred, heated to 30~55°C and reacted for 0.5~2 hours, added 30~100ml of purified water, stirred, collected the organic layer, filtered the solid with suction and dried to obtain ethyl acetyl ferulate. Its synthetic reaction formula is as follows:
[0031] C 12 h 15 o 4 (Ethyl ferulate) + C 4 h 6 o 3 (Acetic anhydride) → C 2 h 4 o 2 (Acetic acid) +C 14 h 17 o 5 (Ethyl Acetyl Ferulate)
[0032] CH 3 COOH (acetic acid) + Na 2 CO 3 (sodium carbonate) → NaHCO 3 (sodium bicarbonate) + CH 3 COONa (sodium acetate).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com