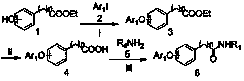Series of diphenyl oxide derivatives and use of diphenyl oxide derivatives in preparation of anti-tuberculosis drugs
A technology of biphenyl ether and tuberculosis, applied in the field of chemical medicine, can solve the problems of long treatment cycle, poor effect and complexity of immunosuppressed patients, and achieve good effect of inhibiting activity in vitro
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Example 1 : the preparation of ethyl 3-phenoxyphenylacetate (3a)
[0018]
[0019] Weigh 10.80g of ethyl m-hydroxyphenylacetate (1a), dissolve it in 100mL of dioxane, add 5.60mL of iodobenzene (2a), 4.76g of cuprous iodide, 7.73g of N,N-dimethylglycine, carbonic acid Cesium 32.58g, heated up to 100°C under the protection of nitrogen, stopped the reaction after 24 hours of reaction, removed the solvent by rotary evaporation, added water and ethyl acetate to separate the layers, extracted the water layer with ethyl acetate three times, combined the organic layers, washed once with saturated brine, Add anhydrous sodium sulfate to dry, concentrate and purify by column chromatography to obtain 10.15 g of light yellow oily substance (3a) with a yield of 79.2%. 1 H-NMR (400MHz, CDCl 3 - d 6 ) δ : 7.31-7.35 (m, 2H), 7.27 (t, J = 8.0 Hz, 1H), 7.10 (t, J = 7.6 Hz, 1H), 7.00-7.02 (m, 3H), 6.95 (s, 1H), 6.90 (dd, J = 8.0, 2.0 Hz, 1H), 4.14 (qua, J = 7.2 Hz, 2H), 3...
Embodiment 2
[0020] Example 2 : the preparation of 3-phenoxyphenylacetic acid (4a)
[0021]
[0022] Weigh 6.41 g of ethyl 3-phenoxyphenylacetate (3a), add 5 g of KOH and 100 mL of water, heat to reflux for 5 hours, stop the reaction, adjust the pH to 2 with concentrated hydrochloric acid, at this time a large amount of light yellow solid powder is precipitated, Filter and wash with water until neutral, and dry in vacuo to obtain 5.43 g of light yellow solid 3-phenoxyphenylacetic acid (4a), with a yield of 95.3%. 1 H-NMR (400MHz, CDCl 3 - d 6 ) δ : 7.75-9.82 (br, 1H), 7.33-7.37 (m, 2H), 7.29 (t, J = 8.0 Hz, 1H), 7.12 (t, J = 7.6 Hz, 1H), 7.01-7.03 (m, 3H), 6.96 (s, 1H), 6.92 (dd, J = 8.0, 2.0 Hz, 1H), 3.63(s, 2H).
Embodiment 3
[0023] Example 3: Preparation of N-(3,4-dichlorophenyl)-2-(3-phenoxyphenyl)acetamide (6a)
[0024]
[0025] Weigh 2.28g of 3-phenoxyphenylacetic acid (4a), add 20mL of anhydrous chloroform, 15mL of thionyl chloride, heat to reflux for 2 hours, evaporate the solvent under reduced pressure, add anhydrous toluene 5mL after mixing, Continue to pressurize and evaporate to dryness to obtain a yellow oil. Dissolve the obtained yellow oil in 5 mL of anhydrous dichloromethane, add 1.74 mL of triethylamine and 1.77 g of 3,4-dichloroaniline in 10 mL of dichloromethane solution under ice cooling, stir at room temperature for 8 h, stop the reaction, and use 20 mL×3 washed three times with water, washed once with saturated brine, dried by adding anhydrous sodium sulfate, filtered, and spin-dried to obtain a yellow solid, purified by column chromatography to obtain a white solid N-(3,4-dichlorophenyl)-2 -(3-phenoxyphenyl)acetamide (6a) 1.86g, yield 50.13%. Melting point: 132.9-133.6 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com