Half antigen and antigen of tenuazonic acid and preparation method and application thereof
A technology of alternarinic acid and hapten, applied in chemical instruments and methods, antifungal/algae/lichen immunoglobulin, animal/human protein, etc. Large-scale screening and other issues, to achieve the effect of high accuracy, high sensitivity, and broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Preparation and Identification of Alternatus Acetic Acid Hapten
[0051] 1. Synthesis of Alternaria ketoacid haptens TeAH and TeAHGA
[0052] Add 30ml of absolute ethanol that has been dehydrated and dried into the three-neck flask, add 10mmol of isoleucine, and add 15ml of thionyl chloride. Reflux at 80°C for 3-4h, cool to room temperature, and concentrate in vacuo at 60°C to remove excess thionyl chloride. Dissolve it in dehydrated ethanol, add 12 mmol of newly prepared sodium ethoxide dropwise under stirring, and react for 30 minutes. Under stirring at 0-4°C, 12 mmol of diketene was added dropwise, and the drop was completed within 30 minutes, and then stirred and reacted at room temperature for 4 hours. 20ml of benzene was added, and 12mmol of newly prepared sodium ethoxide was added dropwise, and the mixture was stirred and refluxed for 3h. After cooling to room temperature, concentrate in vacuo at 60°C to remove the solvent, add dilute hydrochloric acid to adju...
Embodiment 2
[0066] Preparation and Identification of Alternarinic Acid Antigen
[0067] 1. Preparation of alternarinic acid antigen
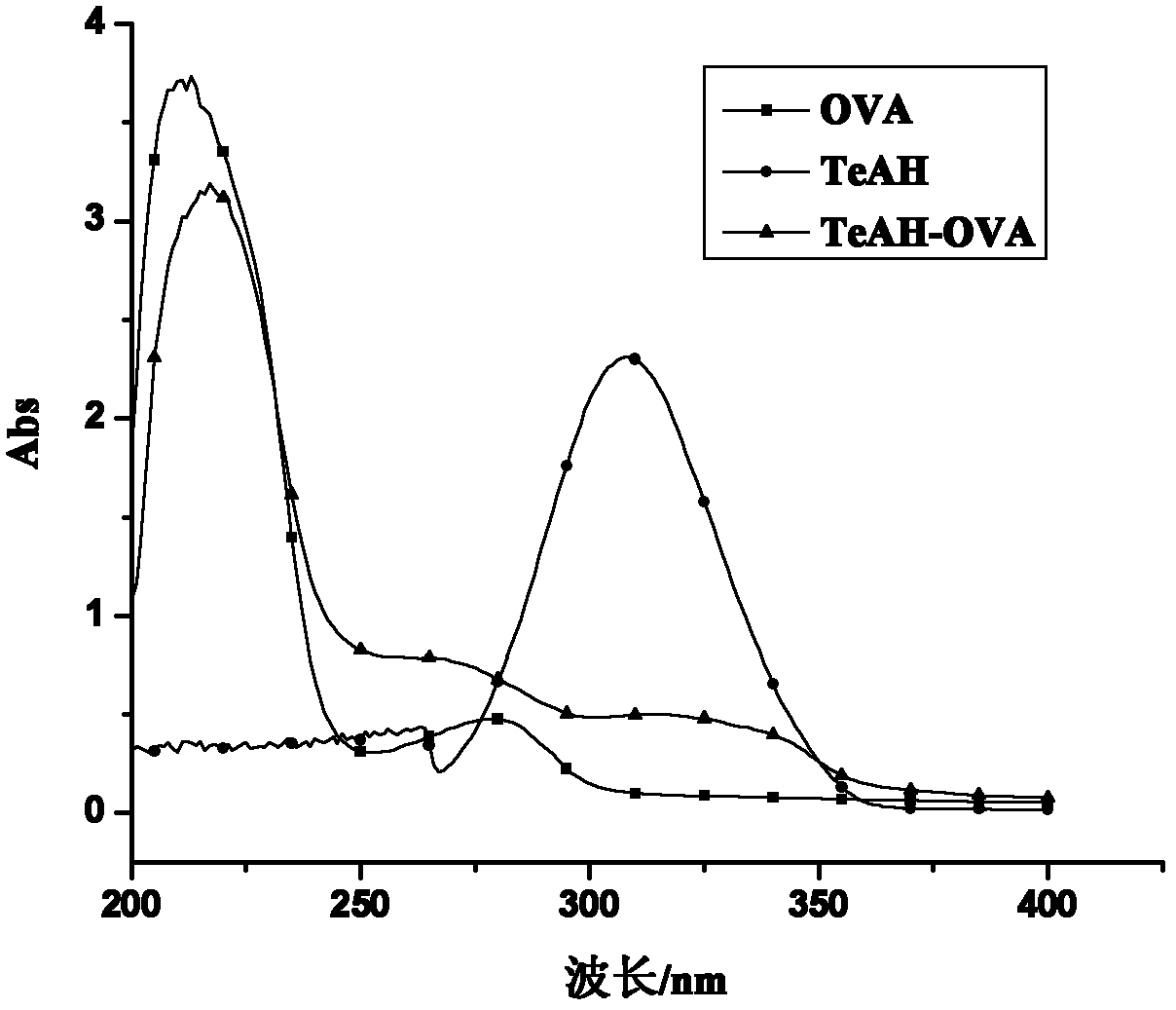
[0068] Glutaraldehyde-linked protein: Weigh 1mmol of the hapten TeAH and 1 / 80mmol of OVA and dissolve them in a PBS solution with a pH value of 8.0, stir well at 25°C, and mix 50-200ul of 25% (volume ratio) ammonium The dialdehyde was added dropwise into the above mixed solution, and after the dropwise addition was completed, the mixture was stirred and reacted at 25° C. for 3 h. After the reaction, put the reaction solution in a dialysis bag and dialyze with normal saline at 4°C for 72 hours, during which the dialysate was changed 6 times to obtain the target product alternarinic acid antigen, which was stored at -20°C after aliquoting.
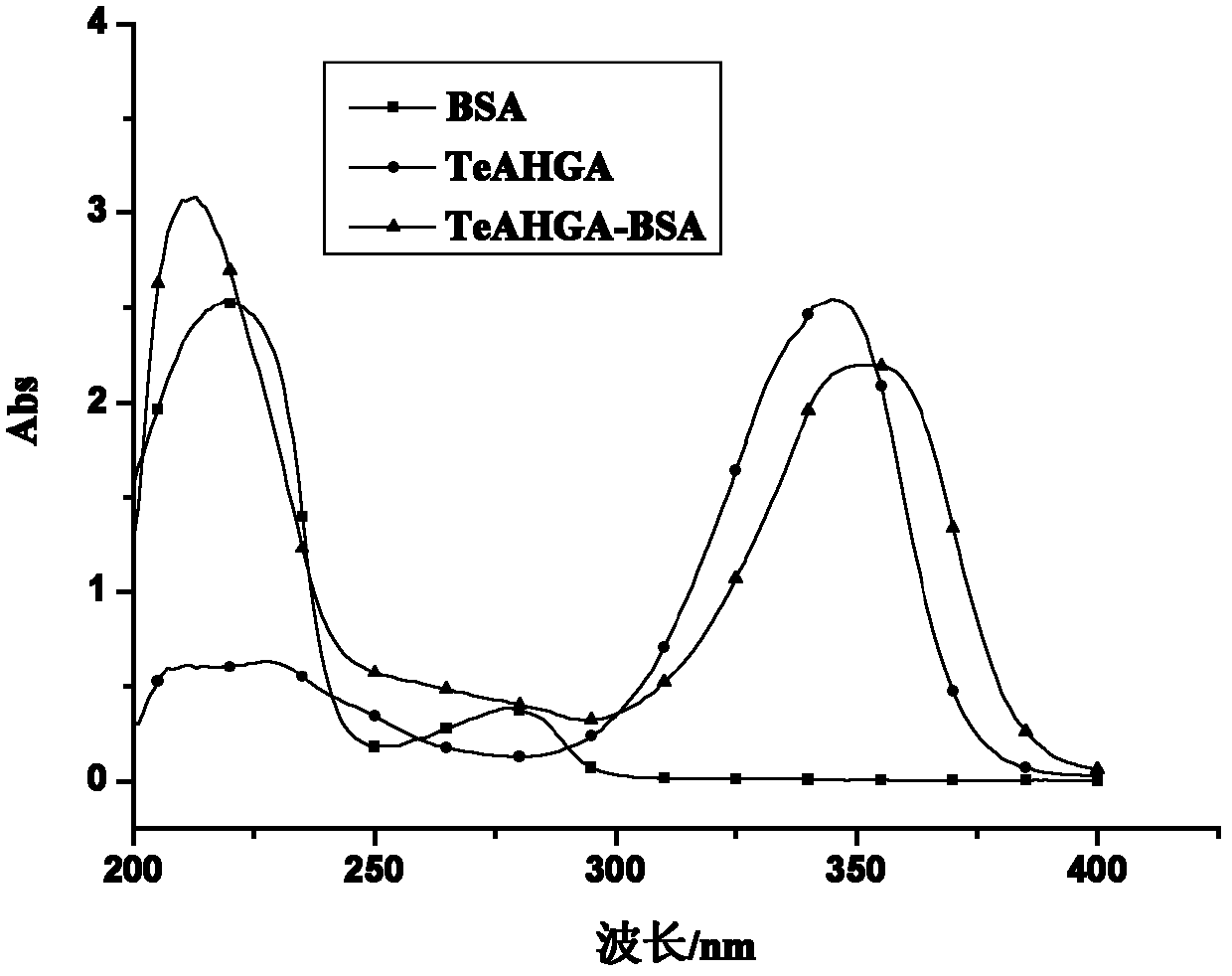
[0069] Active ester fanexin: Dissolve 1 mmol of TeAHGA in DMF, add 3 mmol of DCC and 4 mmol of N-hydroxysuccinimide (NHS) with stirring. Magnetic stirring was carried out overnight at 4°C. After centrifugation, the supe...
Embodiment 3
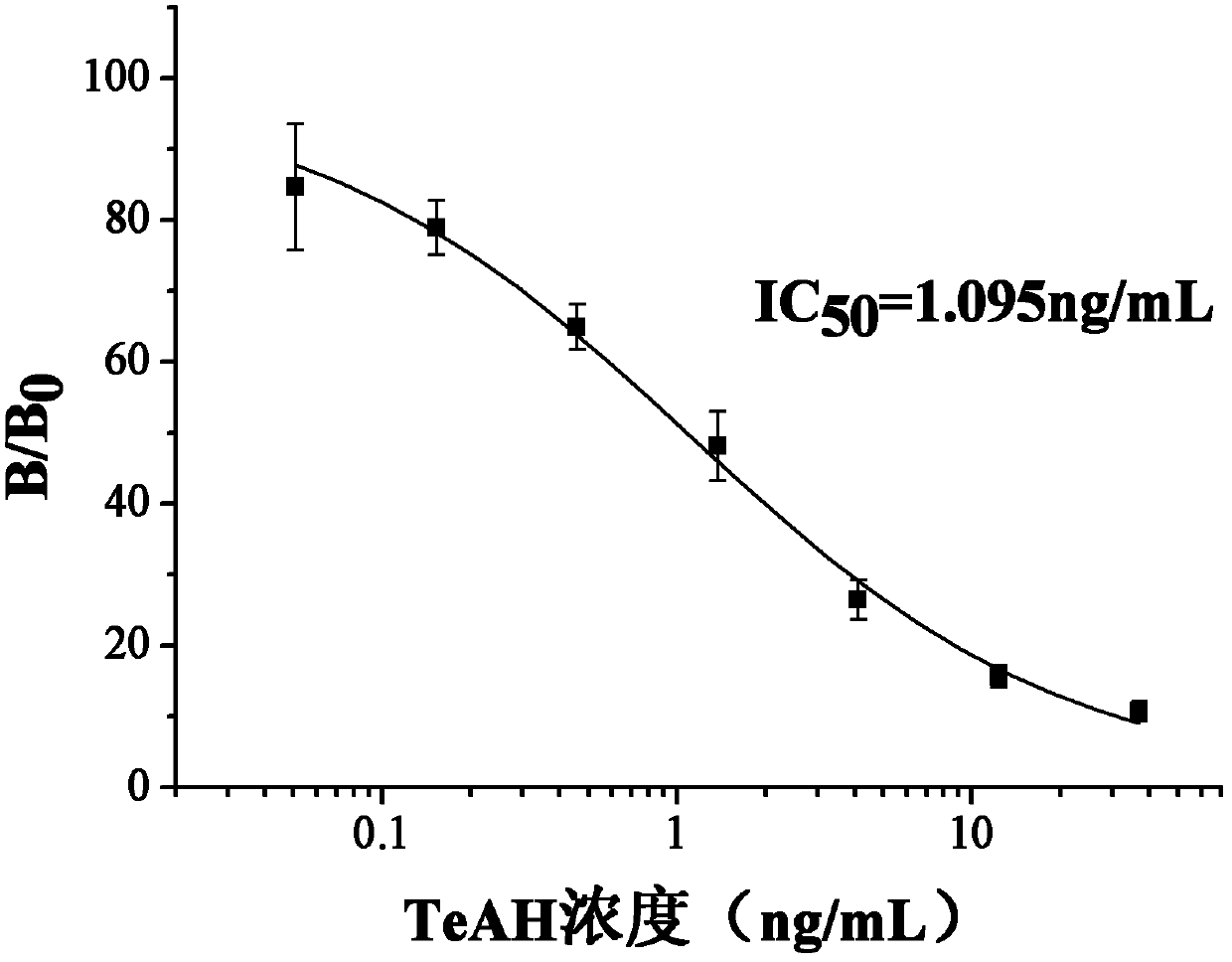
[0074] Detecting the effect of the antigen of the present invention by the alternarinic acid antibody
[0075] 1. Preparation of Alternarinic Acid Antiserum
PUM
| Property | Measurement | Unit |
|---|---|---|
| linear range | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com