MLKL protein and application of MLKL protein as target point of cell necrosis inhibitor
A technology of inhibitors and proteins, applied in the fields of application, peptide/protein components, cells modified by introducing foreign genetic material, etc., can solve problems such as damage expansion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0090] Embodiment 1, the discovery of MLKL protein
[0091] In order to obtain the regulatory protein or substrate protein that interacts with the RIP3 protein, induce cell necrosis and use NSA to prevent cell necrosis downstream of the RIP3 protein, and then use the RIP3 protein to do pull down experiments.
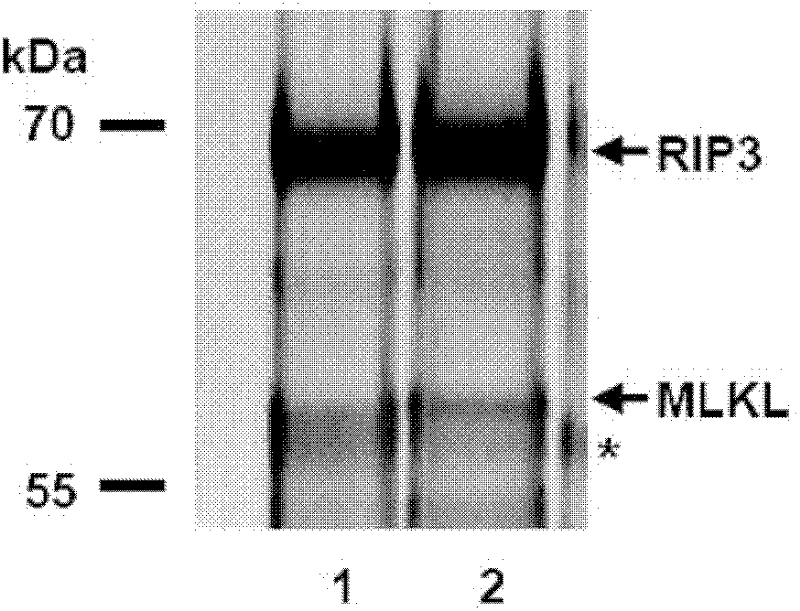
[0092] RIP3-HeLa cells were treated with doxycycline for 24 hours (to induce the expression of RIP3); one group of cells was treated with T / S / Z and NSA (T / S / Z+NSA), and the other group of cells was treated with DMSO (D) ;Harvest the cells and obtain the whole cell extract, perform tandem immunoprecipitation with Flag-HA, then analyze the peptides and the eluted RIP3-binding complex by SDS-PAGE, and perform silver staining. like figure 1As shown, 1 is D, 2 is T / S / Z+NSA, and the asterisk (*) marks the heavy chain of IgG. On the SDS-PAGE denaturing gel, there is a band protein shifted above the heavy chain of 55KD IgG. Therefore, it can be concluded that this protein co...
Embodiment 2
[0094] Interaction between embodiment 2, MLKL protein and RIP3 protein
[0095] 1. On the first day, spread RIP3-HT29 cells on a 96-well plate (5000 cells per well).
[0096] 2. On the second day, they were divided into three groups, and three replicate holes were set for each group, and the following treatments were performed respectively:
[0097] Group 1 (negative control treatment; D): 0.1 μL DMSO was added to each well.
[0098] Group 2 (T / S / Z): TNF-α (T), 100 nM Smacmimetic (S) and 20 μM z-VAD (Z) were added to each well at a final concentration of 20 ng / ml to induce cell necrosis;
[0099] Group 3 (T / S / Z+NSA): Add TNF-α (T), 100 nM Smacmimetic (S) and 20 μM z-VAD (Z) to each well at a final concentration of 20 ng / ml to induce cells Necrosis, while adding NSA to each well (final concentration is 0.5μM);
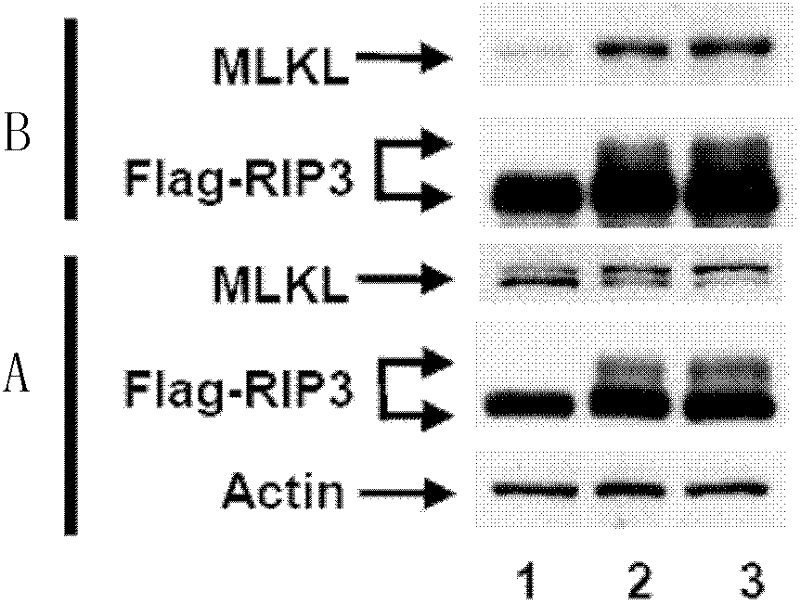
[0100] 3. After the cells treated in step 2 were incubated for 6 hours, the cells were harvested and lysed, and the supernatant was collected. Take 20 μl of supern...
Embodiment 3
[0103] Example 3, Domain analysis of the interaction between MLKL protein and RIP3 protein
[0104] 1. Structural domain analysis
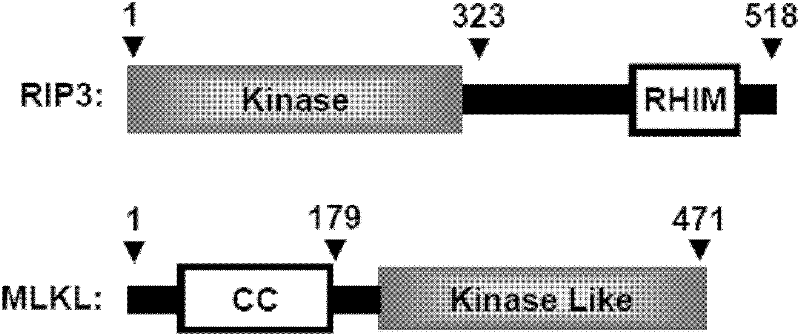
[0105] MLKL protein consists of an N-terminal twisted-coil chain (CC) domain and a C-terminal activation-like domain. The RIP3 protein has an N-terminal kinase domain followed by a RHIM domain, and interacts with the RIP1 protein through RHIM ( Figure 3A ).
[0106] 2. Locating the MLKL protein binding domain of RIP3 protein
[0107] RIP3-FL represents the full-length RIP3 protein (as shown in sequence 3 of the sequence listing). RIP3-Kinase represents the kinase domain of the RIP3 protein (as shown in the 1st to 323rd amino acid residues from the N-terminal of the sequence 3 in the sequence listing). RIP3-K50A represents a mutant RIP3 protein without kinase activity (the full-length RIP3 protein shown in Sequence 3 of the Sequence Listing is mutated from lysine to alanine from the 50th amino acid residue at the N-terminal). MLKL-FL represen...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com