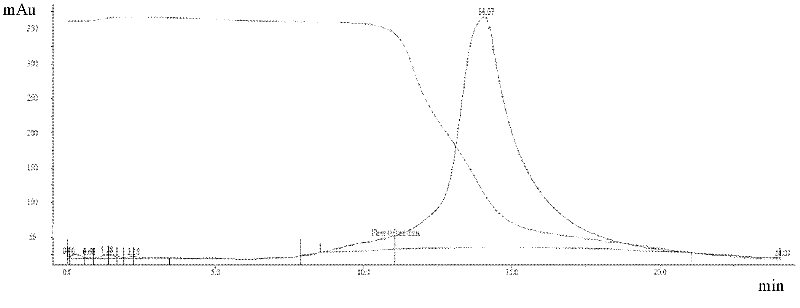
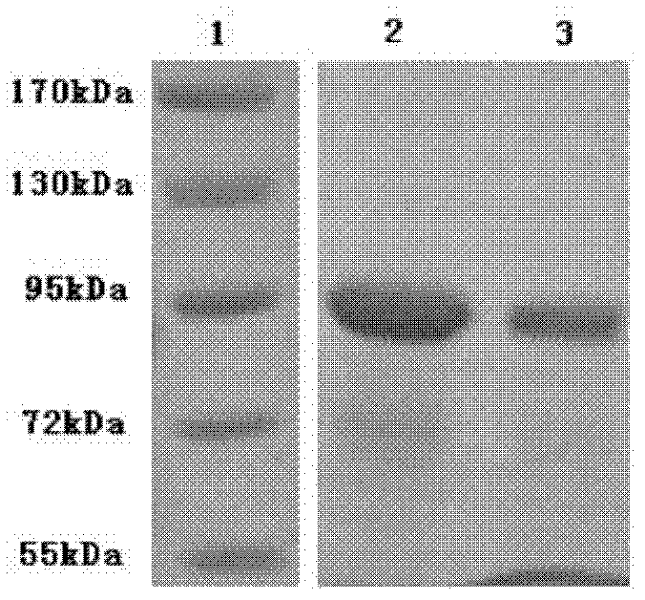
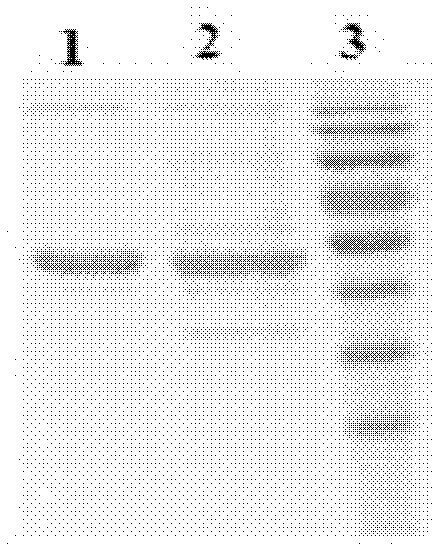
CMG 2mutant and Fc fusion protein, its encoding gene and its application
A technology that encodes genes and proteins, applied in gene therapy, antibodies, hybrid peptides, etc., can solve the problems of antibody loss of antigen affinity, difficulty of mouse-derived antibodies, and inability of antibodies to function, and achieve the effect of long half-life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Example 1, CMG2 mutant gene design and vector construction
[0047] pIRES2-EGFP-CMG2(E117Q)-Fc is obtained by inserting the CMG2-Fc(E117Q) coding gene (sequence 1 in the sequence list) between XhoI and BamHI of pIRES2-EGFP (Clontech, catalog number: 6029-1) Carrier. The specific construction process is as follows:
[0048] The gene vector pIRES2-EGFP-CMG2-Fc containing the 34-225AA extracellular region of CMG2 is used to insert the CMG2-Fc protein (the amino acid sequence of which is shown in the sequence table 4, and the nucleotide sequence of the sequence 3 in the sequence table) into pIRES2-EGFP (Clontech, catalog number: 6029-1) The vector obtained between XhoI and BamHI. The signal peptide of CMG2-Fc protein is the signal peptide sequence of prourokinase. The 117 position Glu(E) is mutated to Gln(Q), and the 158 is Tyr(Y) to Gln(Q). The mutant is The primers are shown in Table 1. The specific test operations are as follows:
[0049] Using pIRES2-EGFP-CMG2-Fc as a tem...
Embodiment 2
[0060] Example 2. Application of CMG2(E117Q)-Fc
[0061] 1. Protein expression and identification
[0062] 1. Transfection of recombinant mutant gene plasmid into CHO-K1 cells and screening of positive clones
[0063] Transfection uses Lipofectamine produced by Invitrogen TM 2000 cationic liposome transfection kit. According to the kit instructions, the CHO-K1 cells transfected with the vector pIRES2-EGFP-CMG2(E117Q)-Fc obtained in Example 1 (Cell Bank of the Type Culture Collection Committee of the Chinese Academy of Sciences, catalog number: GNHa 7) were cultured in 5% CO 2 , 37℃ incubator. Twelve hours after transfection, the culture was replaced with D-MEM / F-12 medium (Gibco, product number 12500-096) containing 10% peptide bovine serum (Hangzhou Sijiqing company, product catalog number: 4033). After 48 hours, D-MEM / F-12 medium containing 750ug / ml G418 and 10% peptide bovine serum (invitrogen, Product catalog number: 12500-096) Continue to culture, change the medium every 2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com