Stable liquid medicinal composition
A light-stable, injection technology, applied in the fields of drug combination, drug delivery, blood diseases, etc., can solve the problems of product safety, high temperature instability, poor water solubility, etc., to improve product quality, simplify prescription composition, reduce The effect of production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
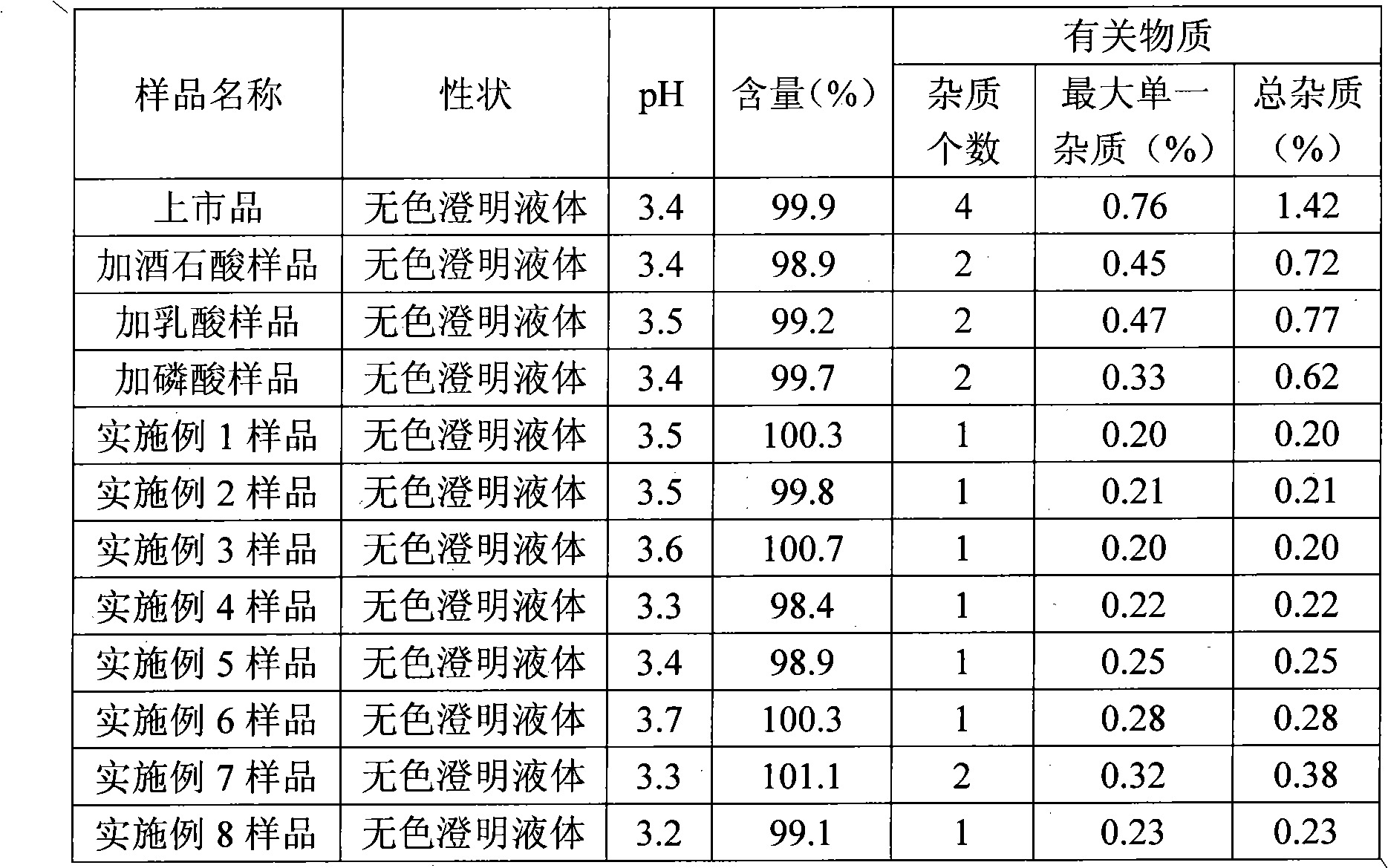
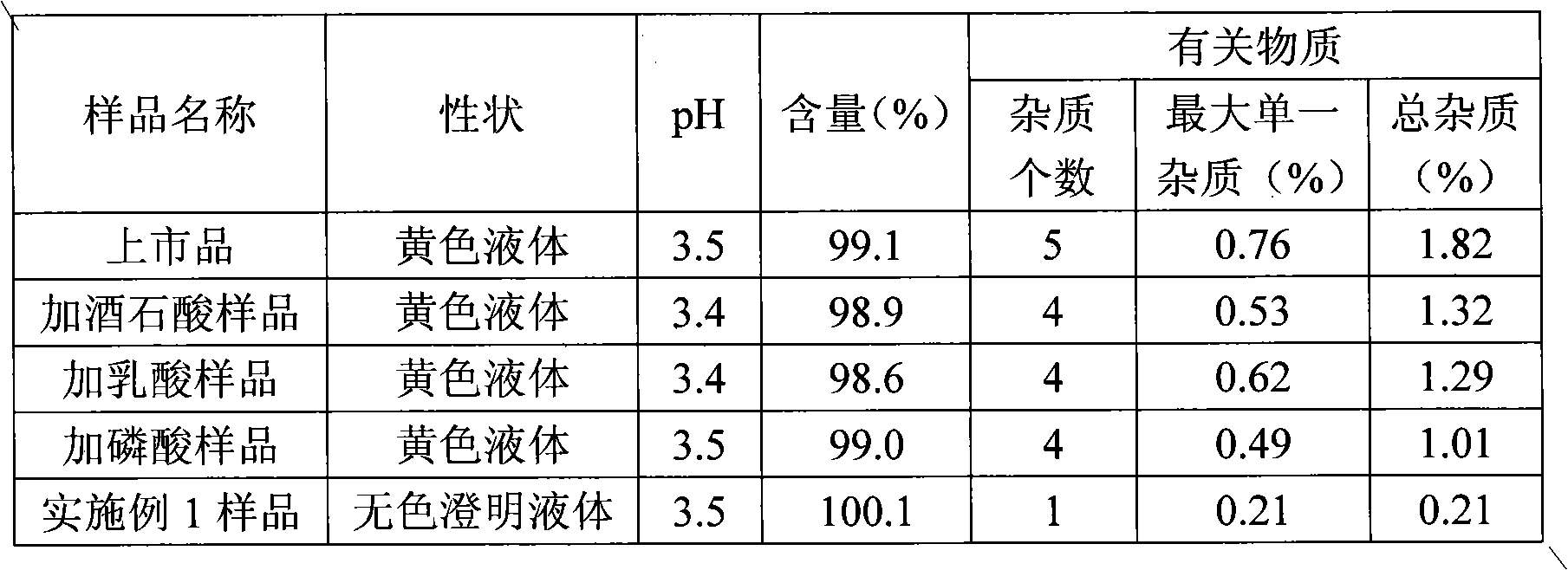
[0039] Preparation of samples for comparative study: Take 1400ml of water for injection, add tartaric acid to adjust the pH to 2.0, add 10g of vinpocetine, stir to dissolve, add 1mol / l NaOH aqueous solution to adjust the pH to 3.0-4.0, add water to a sufficient amount, add injection-grade Activated carbon (0.1%, w / v) was incubated and stirred at 60°C for 30 minutes, filtered, filled in colorless transparent glass ampoules, 2ml each, and sterilized at 121°C for 15 minutes.
[0040] Separately take lactic acid and phosphoric acid to prepare samples in the same way as above, and set aside.
Embodiment 1
[0042] prescription:
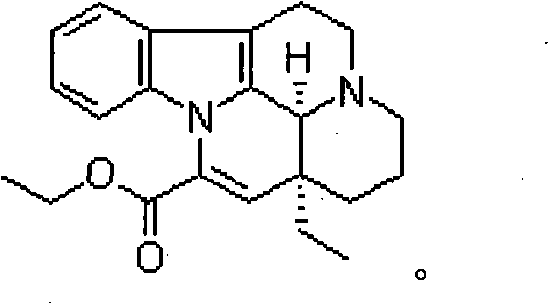
[0043] Vinpocetine 10g
[0044] 1mol / l hydrochloric acid 200ml
[0045] Water was added to 2000ml.
[0046] Preparation method: add hydrochloric acid into water and stir evenly to obtain a hydrochloric acid solution with a volume of about 70% of the prepared volume, add vinpocetine, stir to dissolve it completely, add 1mol / l NaOH aqueous solution to adjust the pH to 3.0-4.0, and replenish Add water to a sufficient amount, add injection-grade activated carbon (0.1%, w / v), insulate and stir at 60°C for 30 minutes, filter, fill in colorless transparent glass ampoules, 2ml each, and sterilize at 121°C for 15 minutes. That is, Vinpocetine Injection.
Embodiment 2
[0048] prescription:
[0049] Vinpocetine 10g
[0050] 1mol / l hydrochloric acid 160ml
[0051] Water was added to 2000ml.
[0052] Preparation method: add hydrochloric acid into water and stir evenly to obtain a hydrochloric acid solution with a volume of about 70% of the prepared volume, add vinpocetine, stir to dissolve it completely, add 1mol / l NaOH aqueous solution to adjust the pH to 3.0-4.0, and replenish Add water to a sufficient amount, add injection-grade activated carbon (0.1%, w / v), insulate and stir at 60°C for 30 minutes, filter, fill in colorless transparent glass ampoules, 2ml each, and sterilize at 121°C for 15 minutes. That is, Vinpocetine Injection.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com