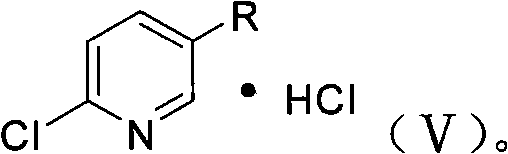
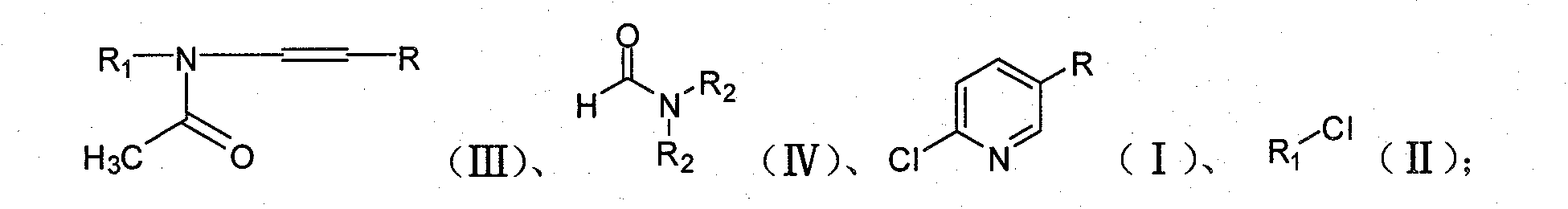
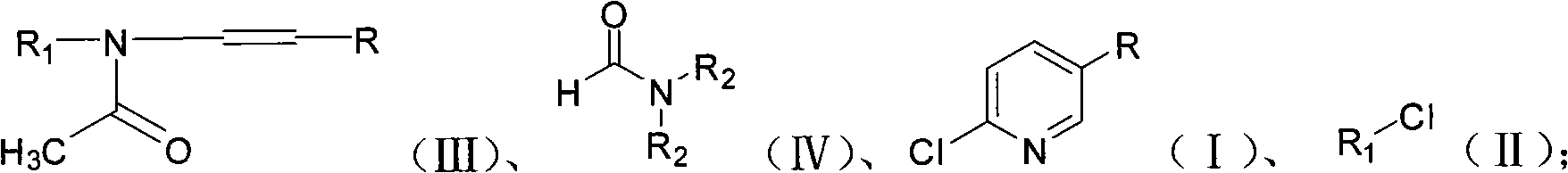Method for preparing 2-chloro-5-substituted pyridine
A technology for pyridine and chlorination reagents, applied in the field of preparation of 2-chloro-5-substituted pyridines, which can solve the problems of high energy consumption, low yield, complicated operation, etc., and achieve simplified operation procedures, reduced production costs, and shortened reaction time Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] In 50ml chlorobenzene, add 45.4g (0.24mol) N-benzyl-N-(1-propenyl) acetamide (self-made, method is referred to Journal of the Chemical Society, Perkin Transactions 1: Organic and Bio-Organic Chemistry (1972 -1999) (1984), (6), 1173-82) and 24.1 g (0.33 mol) of N, N-dimethylformamide, and the temperature was controlled at 15° C. during the addition. After the addition of the materials, the temperature was raised to 30° C., and 71.3 g (0.24 mol) of bis(trichloromethyl)carbonate in chlorobenzene was slowly added dropwise to the system within 1 hour. After the addition, start the distillation reaction at a pressure of 60 mbar, and basically steam the chlorobenzene at 70 ° C, then continue heating to steam the mixture of benzyl chloride and 2-chloro-5-picoline, the distillation reaction process is about 60 minutes to the basic No distillate was evaporated, and the reaction was completed to obtain 63.0 g of the mixture.
[0038] Add 50 g of 35% concentrated hydrochloric acid t...
Embodiment 2
[0040] Add 45.4g (0.24mol) N-benzyl-N-(1-propenyl) acetamide and 24.1g (0.33mol) N,N-dimethylformamide to 50ml chlorobenzene at 15°C to keep the internal The temperature was 15°C, and 71.3 g (0.24 mol) of bis(trichloromethyl)carbonate in chlorobenzene was slowly added dropwise to the system within 1 hour. After the addition, keep the pressure at 20mbar and distill the reaction until the solvent is basically distilled at 50°C, then continue to heat to distill the mixture of benzyl chloride and 2-chloro-5-picoline, and distill for about 30 minutes until there is basically no distillate. , the reaction was completed, and 68.0 g of the mixture was obtained.
[0041] Add 50 g of 35% concentrated hydrochloric acid to the above mixture, stir vigorously for 15 minutes, and then let stand to separate layers. The upper layer (oil layer) was separated to obtain 29.5 g of benzyl chloride, and the content of GC analysis was 97.5%; the acid solution in the lower layer was adjusted to pH 9 ...
Embodiment 3
[0043] Addition operation is with embodiment 1. After the addition, the distillation reaction was carried out under normal pressure. When the temperature of the reaction solution reaches 140° C., the chlorophenyl is basically evaporated and removed. Continue heating to distill out the mixture of benzyl chloride and 2-chloro-5-picoline. Distill the reaction for about 90 minutes until almost no distillate comes out. After the reaction is completed, 58.0 g of the mixture is obtained.
[0044] The operation of separating product and by-product from the mixture is the same as in Example 2. 22.0 g of the product 2-chloro-5-methylpyridine was obtained, with a content of 97.8%, and a yield of 70.3%; 23.3 g of by-product benzyl chloride, with a content of 97.9% by GC analysis.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com