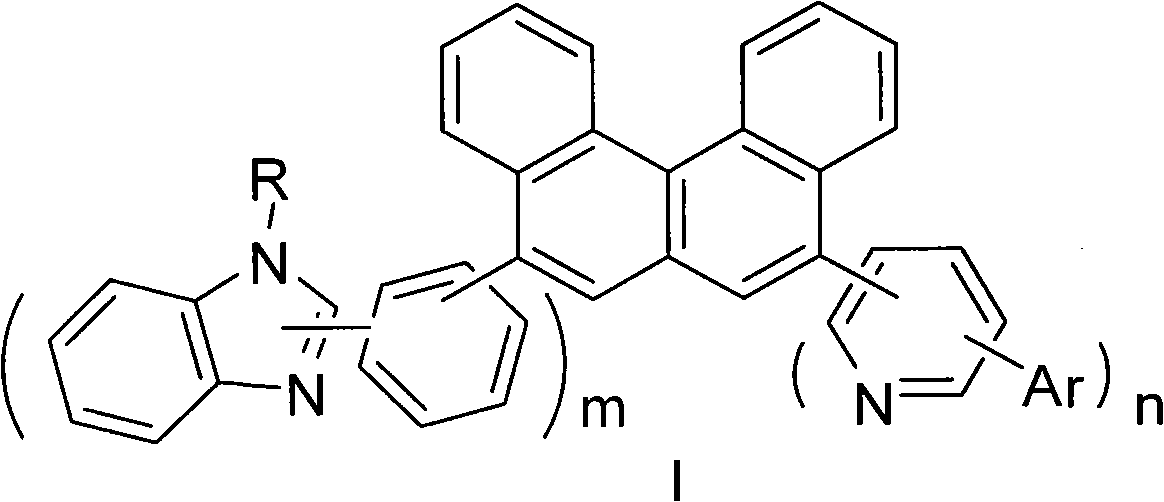
Benzophenanthrene compound containing benzoglioxaline group and application thereof
A technology of organic compounds and compounds, applied in organic chemistry, electrical components, circuits, etc., can solve problems such as short life of blue phosphorescent devices, suppression of heat-absorbing energy transfer process, and reduced device efficiency, so as to improve electron transport performance and high Good electron transport performance and film-forming properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
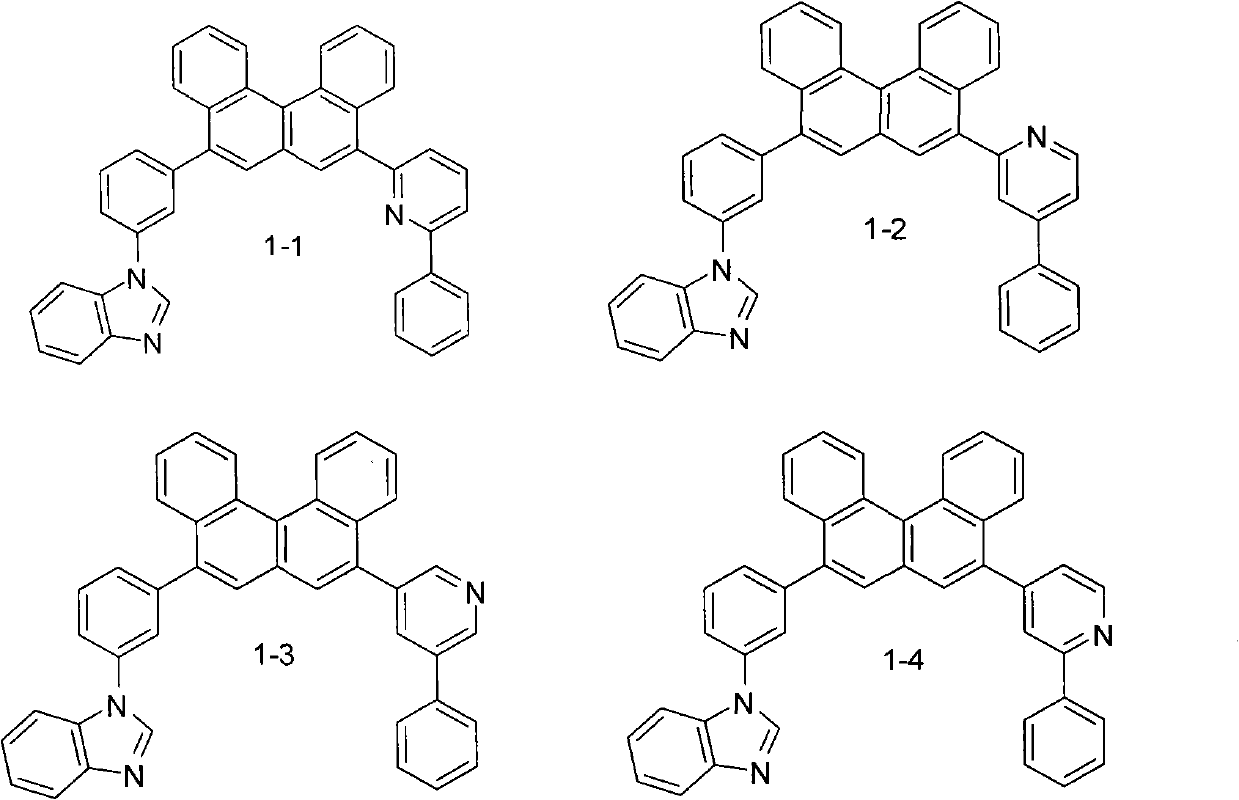
[0039] Example 1 Preparation of Compound 1-1
[0040]
[0041] 11.8g (0.10mmol) of benzimidazole was dissolved in 10.1mL of triethylamine, 28.1g (0.10mmol) of isobromoiodobenzene was added dropwise at zero degree, stirred at room temperature for 30 minutes and then heated to 70-80°C and stirred for 2 hours. The reaction was quenched with water, extracted with dichloromethane, dried with magnesium sulfate, and recrystallized from methanol to obtain 23.8g of compound A. Dissolve A in dry THF, add n-butyl oligomer at -80°C, stir for 15min, and add three Isopropyl borate. After hydrolysis, adjust the pH to neutral and precipitate compound B. 20.2g. A 1:1 coupling reaction between B and 5,8-dibromobenzene[c]phenanthrene was carried out to obtain intermediate C, and C and compound E were reacted to obtain compound 1-1. Purified by column chromatography, the eluent is petroleum ether: dichloromethane = 2:1. MS(m / e): 573, elemental analysis (C 42 H 27 N 3 ): Theoretical value C: 87.93...
Embodiment 2-30
[0046] Examples 2-30 are all similar to Example 1. Intermediate C reacts with pyridine boronic acid substituted with different aryl groups to obtain the target product. The details are as follows:
Embodiment 2
[0047] Example 2 Synthesis of Compound 1-2
[0048] Using intermediate C and 6-phenyl-2-pyridineboronic acid as raw materials, compound 1-2 is obtained. MS(m / e): 573, elemental analysis (C 42 H 27 N 3 ): Theoretical value C: 87.93%, H: 4.74%, N: 7.32%; measured value C: 87.90%, H: 4.81%, N: 7.29%. The yield was 61.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com