Gramicidin, isomer thereof and application thereof
A short peptide and structural formula technology, applied in the field of its isomers and short peptides, can solve the problems of limiting biological activity and concentration drop
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
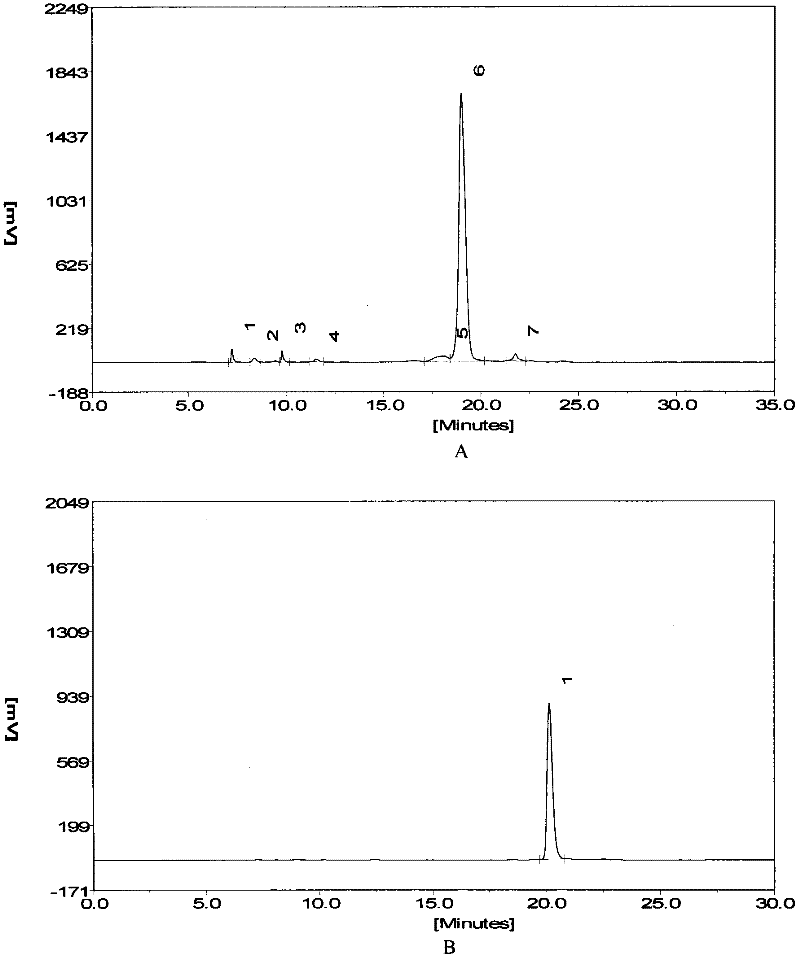
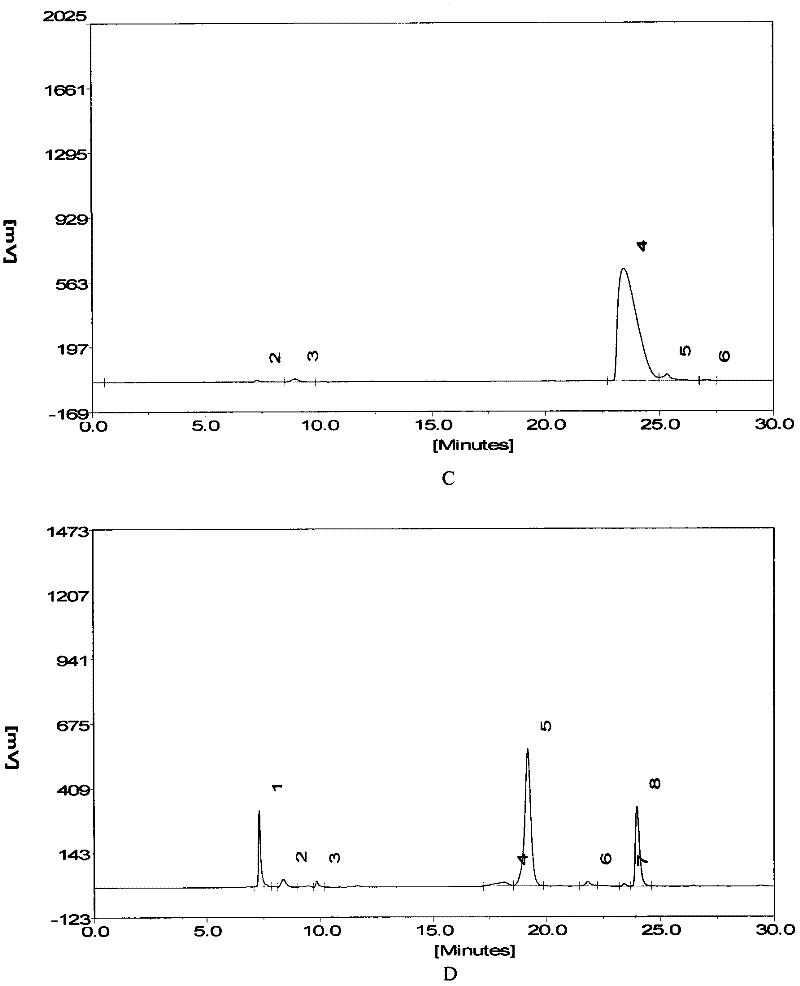
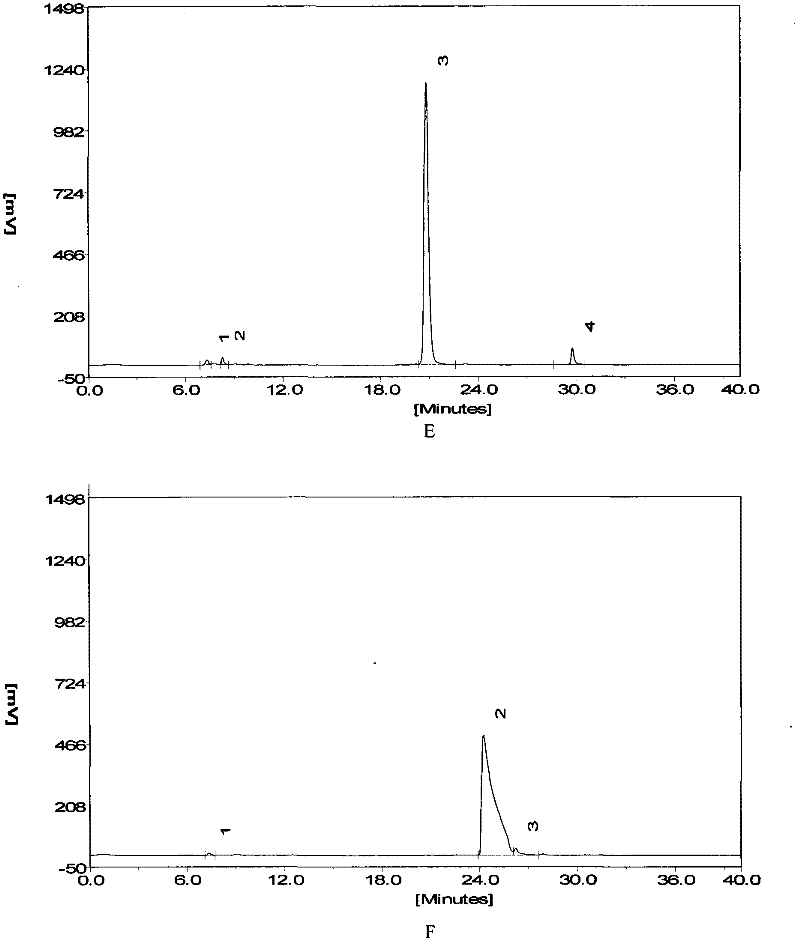
[0042] Implementation 1 The enzymolysis resistance effect of short peptides of the present invention and its isomers compared to angiotensin 1-7
[0043] Dissolve angiotensin 1-7, inventive short peptide 1, and inventive short peptide 2 to 5 mmol / L with PBS.
[0044] Use PBS to dissolve angiotensin 1-7, inventive short peptide 1, and inventive short peptide 25 μl + 20 μl PBS in a 37°C water bath for 30 minutes + 5 μl acetonitrile for standard spectra by HPLC.
[0045] Invention short peptide 15μl + 10μl ACE or NEP or LAP enzyme (0.05U) + 10PBS 37°C water bath for 30min + 5μl acetonitrile for HPLC.
[0046] Invention short peptide 25 μl + 10 μl ACE or NEP or LAP enzyme (0.05 U) + 10PBS 37°C water bath for 30 min + 5 μl acetonitrile for HPLC.
[0047] Chromatographic conditions
[0048] Liquid A: 0.1% phosphoric acid solution
[0049] Solution B: 100% acetonitrile solution
[0050] C 18 column
[0051] Flow rate: 0.35ml / min
[0052] After equilibrating the column with 12%...
Embodiment 2
[0054] Example 2 Short peptides of the present invention and their isomers stimulate HUVEC to release nitric oxide
[0055] Digest HUVEC cells with 2×10 per well 5 Cells were plated into a 12-well plate and added with 10% FBS DMEM at 37°C 5% CO 2 Incubate overnight.
[0056]Take 10% FBS DMEM to dilute ang1-7 and the short peptide of the present invention respectively, its structural formula is Ac-Asp-Arg-Val-Tyr-Ile-His-Pro-NH 2 , and Ac-Asp-Arg-Val-Tyr(D)-Ile-His(D)-Pro-NH 2 , is 1μm / L. The 12 wells were divided into drug-free group, ang1-7, short peptide 1 of the present invention, and short peptide 2 of the present invention. Two sub-wells in each of the four groups absorbed the culture solution, added 900 μl of fresh medium or drugs, and incubated at 37°C for 30 minutes.
[0057] Take out the 12-well plate and take 50 μl of culture solution from each well to measure the content of nitric oxide.
[0058] see results figure 2 , as shown in the figure, after adding the...
Embodiment 3
[0059] Example 3 Short peptides of the present invention and their isomers inhibit tubule formation in HUVEC cells
[0060] Pre-cool the 96-well plate and 200μl pipette tips, and melt the matrgiel matrigel stored at -20℃ overnight at 4℃.
[0061] Quickly add 50 μl of melted matrgiel per well to a pre-cooled 96-well plate, and place at room temperature for 10 minutes until the matrgiel solidifies.
[0062] Digest HUVEC cells to adjust the cell density to 2×10 5 144 μl of cell suspension was added to solidified matrgiel.
[0063] DMEM deployment 1μm / L ang1-7, short peptide 1 of the present invention, short peptide 2 of the present invention, 16 wells were divided into four groups: no drug group, ang1-7, short peptide 1 of the present invention, short peptide 2 of the present invention, each group 3 Add 16 μl of drug solution to each auxiliary well in the 96-well plate with cells added.
[0064] 37°C 5% CO 2 After culturing for 8 hours, the effect of tubule formation was obse...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com