Plasmid for heterologous protein solubility expression and preparation and application method thereof
A technology of exogenous protein and application method, which is applied to the plasmid for soluble expression of exogenous protein and the fields of its preparation and application, and can solve the problems of the effect of co-expression folding auxiliary protein and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
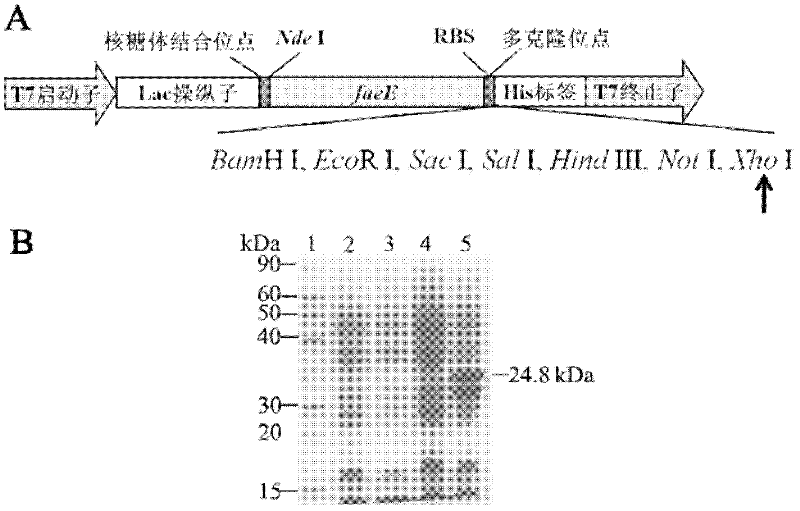
[0045] Schematic diagram of pET30e expression vector in Escherichia coli and SDS-PAGE analysis of expression of molecular chaperone FaeE in Escherichia coli
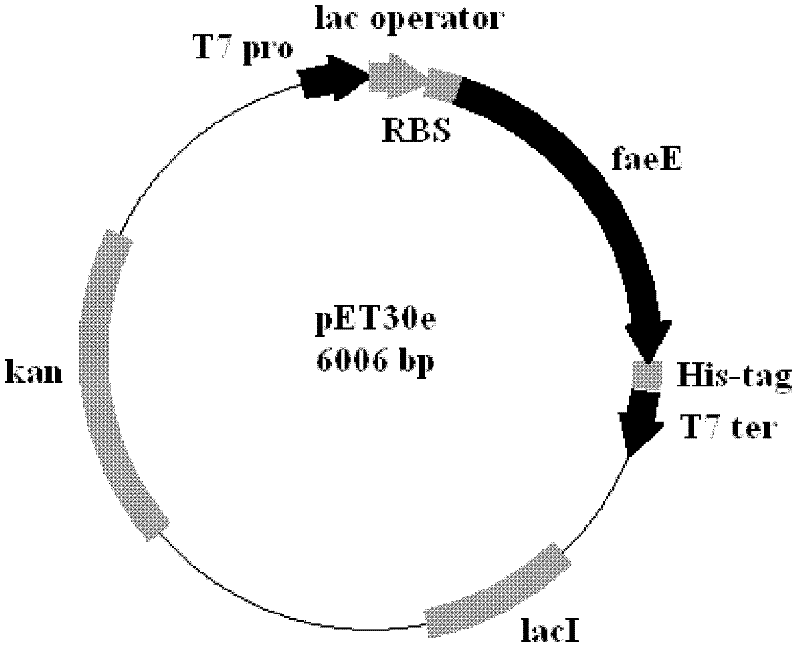
[0046] The molecular chaperone FaeE coding sequence was cloned into the commercialized expression vector pET30a through two restriction enzymes Nde I and Bam HI, so that the gene was located under the control of the T7 promoter and the lac operator. The E. coli ribosome binding site is added to the 5' end, followed by multiple cloning sites that can be used to clone foreign protein genes, including Bam HI, EcoR I, Sac I, Hind III, Not I, Xho I, followed by the multiple cloning site is a 6-histidine coding sequence. Foreign genes are terminated by the T7 terminator. Carrier structure such as figure 1 and figure 2 .
[0047] Transform pET30a and the obtained pET30e E. coli expression vector into human E. coli expression strain BL21(DE3), and single colony of recombinant E. coli pET30a / BL21(DE3) and pET30e / BL21(DE3) in...
Embodiment 2
[0049] SDS-PAGE and Western blot analysis of pET30CTB and pET30eCTB expressed in Escherichia coli.
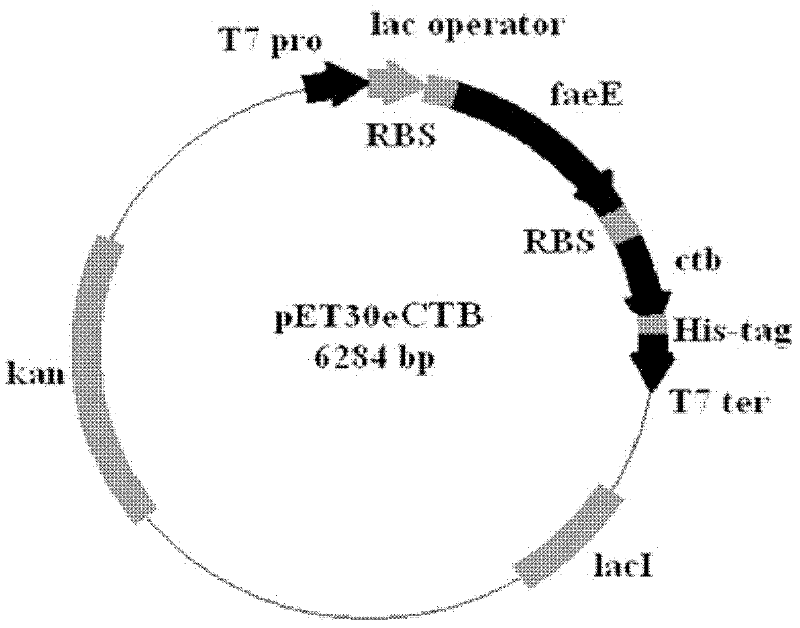
[0050] The coding sequence of the CTB coding sequence was cloned into pET30e treated with the same digestion by Bam HI and Xho I to obtain the expression vector pET30eCTB. The vector structure is as follows: image 3 .
[0051] The DNA sequence of the vector pET30eCTB plasmid is shown in Seq ID No.2, which includes the coding sequence of the molecular chaperone FaeE and the coding sequence of the cholera toxin B subunit CTB, and the cholera toxin B subunit (CTB) coding sequence It was amplified from classical Vibrio cholerae (CVC) genomic DNA as a template.
[0052] Transform pET30CTB and pET30eCTB into Escherichia coli expression strain BL21(DE3) to obtain recombinant Escherichia coli pET30CTB / BL21(DE3) and pET30eCTB / BL21(DE3). The methods of bacterial culture, protein induction and treatment are the same as in Example 1. The protein is separated by SDS-PAGE. After separatio...
Embodiment 3
[0055] SDS-PAGE and Western blot analysis of pET30PRX and pET30EPRX expressed in Escherichia coli
[0056] The coding sequence of PRX was cloned into pET30e treated with the same enzyme digestion by Bam HI and Xho I to obtain the expression vector pET30ePRX, the vector structure is as follows Figure 4 .
[0057] The DNA sequence of the vector pET30ePRX plasmid is shown in Seq ID No.3, comprising the coding sequence of molecular chaperone FaeE and the coding sequence of rice endogenous peroxidase, and the rice endogenous peroxidase (PRX) The gene sequence was amplified using rice genomic DNA as a template.
[0058] Transform pET30PRX and pET30EPRX into Escherichia coli expression strain BL21(DE3) to obtain recombinant Escherichia coli pET30PRX / BL21(DE3) and pET30ePRX / BL21(DE3). The methods for bacterial culture, protein induction and treatment are the same as in Example 1, and the Western blot analysis method is the same as in Example 2. SDS-PAGE results ( Figure 6 A) sho...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com