Preparation of isobutyryl substituted 1,10-phenanthroline complex and application of complex serving as catalyst
A technology of isobutyryl and phenanthroline, applied in 1, the application field of ethylene oligomerization catalyst, can solve the problem of many steps of ligand synthesis and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
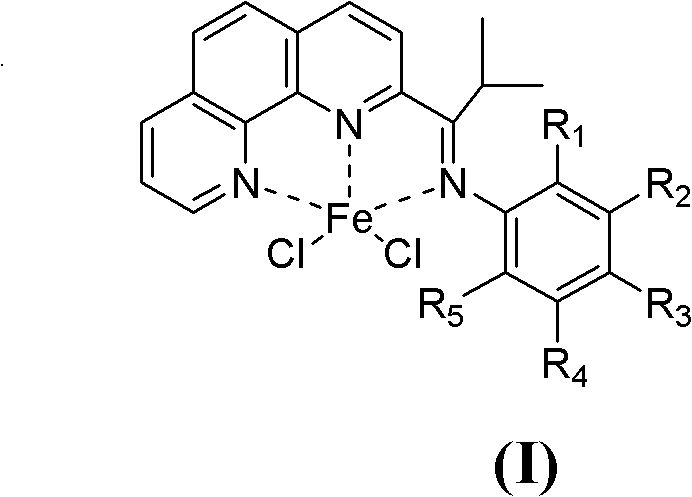
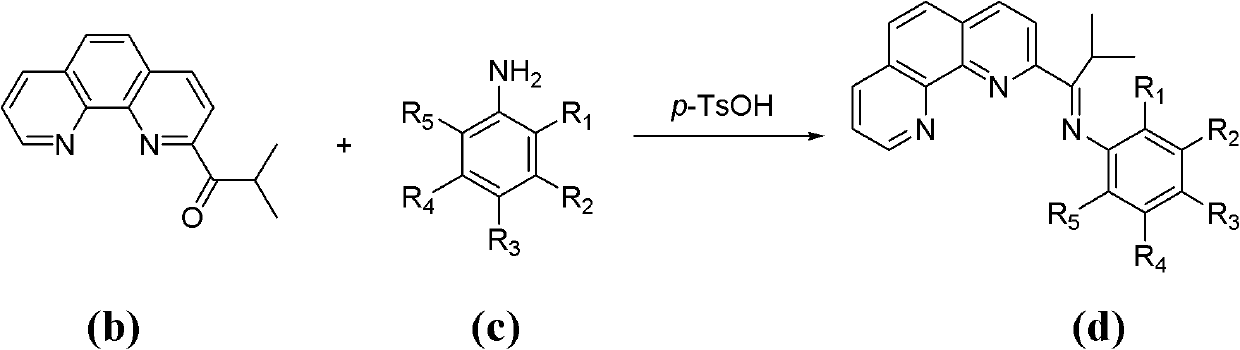
[0038] 1. Synthesis of Catalyst Chloride 2-isobutyryl-1,10-phenanthroline 2,6-diethylanilinate iron (II) complex
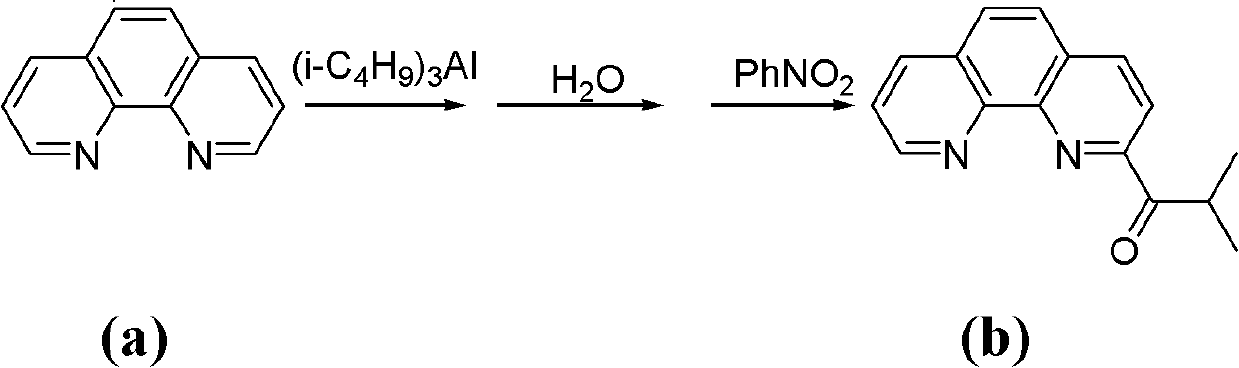
[0039] The synthesis of a.2-isobutyryl-1,10-phenanthroline (see the following reaction scheme)
[0040]
[0041] Put 5.1 g (28.3 mmol) of 1,10-phenanthroline into a 250 ml three-necked flask, and dissolve it with 100 ml of toluene under nitrogen protection and magnetic stirring. Slowly add 13.7ml of triisobutylaluminum (d=0.82g / ml, 56.6mmol) dropwise to the three-necked flask under stirring at -60°C, the dropwise addition is completed in about 15 minutes, and continue stirring at this temperature for 18h. Afterwards, the temperature was raised to about 30° C., and stirring was continued for 10 h. Then the reaction mixture was cooled to about -30°C, 50ml of distilled water was slowly added thereto, and then heated to 30°C and stirred for 10h. Then separate the liquids, take out the organic phase, and extract the inorganic phase three times with dichloromethane...
Embodiment 2-47
[0057] Repeat step 1 of Example 1, except that 2,6-diethylaniline in step b of Example 1 is replaced by the following substituted anilines in turn: 2-methylaniline, 3-methylaniline, 4- Methylaniline, 2,3-dimethylaniline, 2,4-dimethylaniline, 2,5-dimethylaniline, 2,6-dimethylaniline, 3,4-dimethylaniline, 3 , 5-dimethylaniline, 2,4,6-trimethylaniline, 4-bromo-2,6-dimethylaniline, 2-ethylaniline, 2-ethyl-6-methylaniline, 2 -isopropylaniline, 2,6-diisopropylaniline, 2-fluoroaniline, 2-fluoro-4-methylaniline, 2-fluoro-5-methylaniline, 2,4-difluoroaniline, 2 , 5-difluoroaniline, 2,6-difluoroaniline, 3,4-difluoroaniline, 2,3,4-trifluoroaniline, 2,4,5-trifluoroaniline, 2,4,6-trifluoroaniline Fluoroaniline, 2,3,4,5,6-pentafluoroaniline, 3-chloroaniline, 2,6-dichloroaniline, 2,3,4-trichloroaniline, 2,4,5-trichloroaniline, 2,4,6-Trichloroaniline, 2-bromoaniline, 2-bromo-4-methylaniline, 2-bromo-4-fluoroaniline, 4-bromo-2-fluoroaniline, 2,6-dibromoaniline , 2,6-dibromo-4-methylaniline,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com