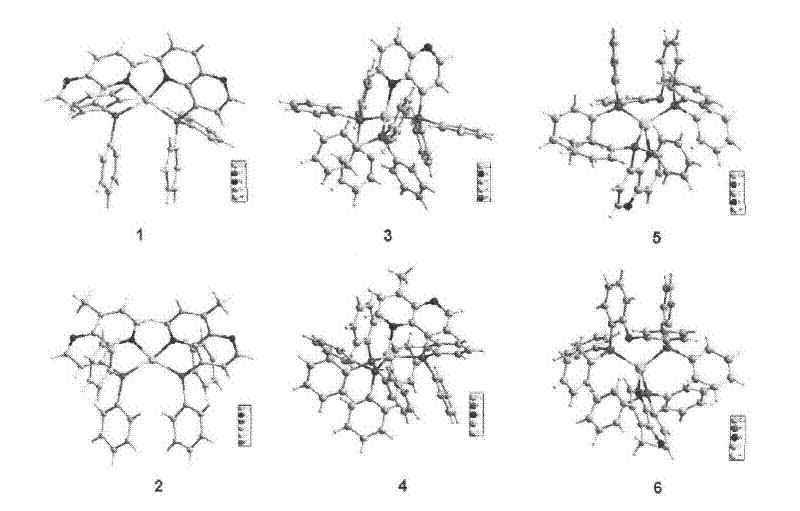
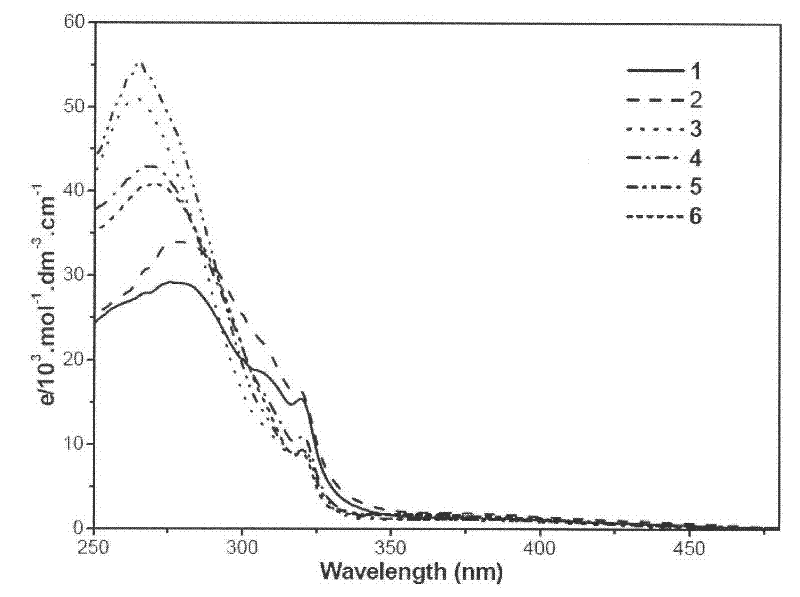
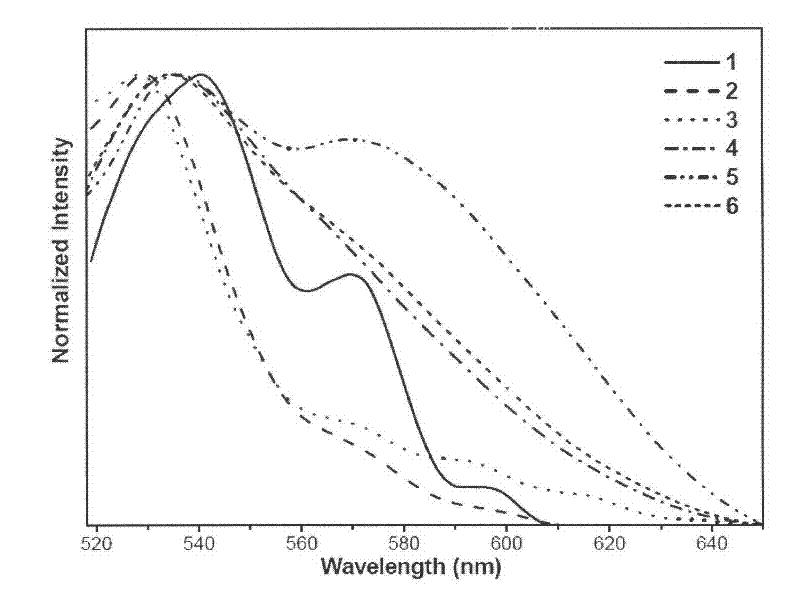
4-phosphino-1,5-naphthyridine derivative copper (i) complex luminescent material and application
A technology of naphthyridine copper and complex, applied in the field of optoelectronic materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1: [Cu(ND) 2 ](PF 6 ) (1) synthesis
[0033] Under the protection of argon, 10mL CH containing ND (0.41g, 1.3mmol) 2 Cl 2 The solution was slowly added to the solution containing [Cu(NCCH 3 ) 4 ](PF 6 ) (0.24g, 0.65mmol) in 30mL methanol solution, stirred at room temperature for 2h and then filtered, and the filtrate was desolventized in vacuo to obtain a red solid. The solid was washed with ether and recrystallized from tetrahydrofuran / methanol / ether (2:1:6 v / v / v) to obtain red crystals [Cu(ND) 2 ](PF 6 ) 0.85g, yield 78%. The crystal structure of the complex is determined by X-ray single crystal diffractometer, and its crystallographic parameters are: space group P21 / C, a=14.386(3), b=23.133(5), c=23.326(5), α= 90.00, β=105.24(3), γ=90.00°,
[0034]
Embodiment 2
[0035] Embodiment 2: [Cu(mND) 2 ](PF 6 ) (2) synthesis
[0036] Under argon protection condition, 10ml CH containing mND (0.33g, 1.0mmol) 2 Cl 2 The solution was slowly added to the solution containing [Cu(NCCH 3 ) 4 ](PF 6 ) (0.19g, 0.5mmol) in 30mL of methanol solution, stirred at room temperature for 2h and then filtered, and the filtrate was desolventized in vacuo to obtain an orange solid. The solid was washed with ether and recrystallized from tetrahydrofuran / methanol / ether (2:1:6v / v / v) to obtain orange crystals [Cu(mND) 2 ](PF 6 ) 0.59g, yield 62%. The crystal structure of the complex is determined by X-ray single crystal diffractometer, and its crystallographic parameters are: space group C2 / C, a=16.242(3), b=17.274(4), c=17.517(4), α= 90.00, β=117.20(3), γ=90.00°,
[0037]
Embodiment 3
[0038] Embodiment 3: [Cu(ND)(PPh 3 ) 2 ](PF 6 ) (3) synthesis
[0039] Under argon protection condition, 15ml CH containing ND (0.55g, 1.75mmol) 2 Cl 2 The solution was slowly added to the solution containing [Cu(NCCH 3 ) 4 ](PF 6 ) (0.65g, 1.75mmol) in 35mL methanol solution, stirred at room temperature for 1h and then added triphenylphosphine (PPh 3 ) (0.92g, 3.5mmol) in 15ml CH 2 Cl 2 The solution was stirred at room temperature for 1 h and then filtered, and the solvent was removed from the filtrate in vacuo to obtain a yellow solid. The solid was washed with ether and recrystallized from tetrahydrofuran / methanol / ether (2:1:6 v / v / v) to obtain yellow crystals [Cu(ND)(PPh 3 ) 2 ](PF 6 ) 1.51g, yield 82.3%. The crystal structure of the complex is determined by X-ray single crystal diffractometer, and its crystallographic parameters are: space group P21 / c, a=12.520(3), b=16.408(3), c=26.583(5), α= 90.00, β=93.58(3), γ=90.00°,
[0040]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com