Gemcitabine hydrochloride lyophilized composition and preparation method thereof
A technology of gemcitabine hydrochloride and composition, applied in the field of gemcitabine hydrochloride freeze-dried composition and preparation thereof, can solve the problems of increased risk of hemolytic reaction, increase product cost, low manufacturing cost, etc., avoid the risk of hemolytic effect, and improve production efficiency , the effect of reducing manufacturing costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
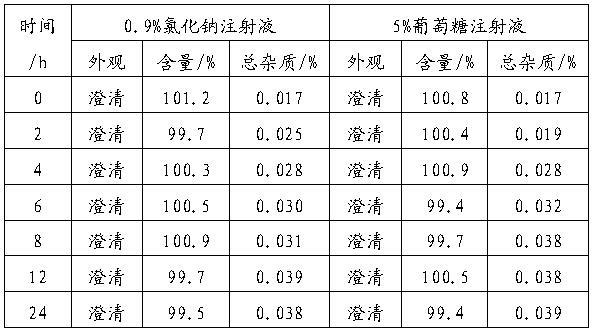
[0046] Example 1 : Preparation of gemcitabine hydrochloride freeze-dried powder injection (the weight ratio of gemcitabine hydrochloride, HP-β-CD, mannitol and sodium acetate is 1:0.45:0.45:0.05; the molar ratio of gemcitabine hydrochloride and HP-β-CD is 1:0.08)
[0047] prescription
[0048] Gemcitabine Hydrochloride 228g HP-β-CD 100g Mannitol 100g Sodium acetate 12.5g Add water for injection to 2500ml Co-made 1000 bottles
[0049] Weigh the raw and auxiliary materials according to the prescription, add water for injection cooled to 25°C, the dosage is 80%, dissolve and stir evenly, adjust the pH to 3.0 with 1mol / L hydrochloric acid or 1mol / L sodium hydroxide solution, and pass the intermediate inspection , add water for injection to make up to the full volume; the solution is filtered through 0.45 μm and 0.22 μm microporous membranes, then sent to the filling room, filled in vials, half-tightened, put on a plate, placed in a fr...
Embodiment 2
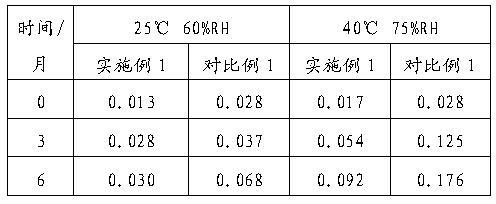
[0051] Example 2 : Preparation of gemcitabine hydrochloride freeze-dried powder injection (the weight ratio of gemcitabine hydrochloride, HP-β-CD, glucose and sodium acetate is 1:0.22:0.45:0.05; the molar ratio of gemcitabine hydrochloride and HP-β-CD is 1:0.04)
[0052] prescription
[0053] Gemcitabine Hydrochloride 228g HP-β-CD 50g glucose 100g Sodium acetate 12.5g Add water for injection to 2500ml Co-made 1000 bottles
[0054] The raw and auxiliary materials of the prescription amount were weighed, and the method was the same as in Example 1 to prepare gemcitabine hydrochloride powder injection for injection.
Embodiment 3
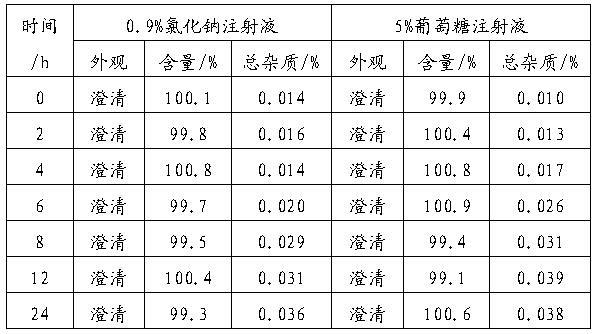
[0055] Example 3 : Gemcitabine hydrochloride freeze-dried powder preparation (the weight ratio of gemcitabine hydrochloride, HP-β-CD, lactose and sodium acetate is 1:0.22:0.45:0.05; the molar ratio of gemcitabine hydrochloride and HP-β-CD is 1:0.04)
[0056] prescription
[0057] Gemcitabine Hydrochloride 228g HP-β-CD 50g lactose 100g Sodium acetate 12.5g Add water for injection to 2500ml Co-made 1000 bottles
[0058] The raw and auxiliary materials of the prescription amount were weighed, and the method was the same as in Example 1 to prepare gemcitabine hydrochloride powder injection for injection.
PUM
| Property | Measurement | Unit |
|---|---|---|
| solubility (mass) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com