Spherical V-MCM-48 catalyst for hydrogen peroxide selective oxidation of phenylethylene oxide and method
A V-MCM-48, catalyst technology, applied in the field of hydrogen peroxide selective styrene oxide, to achieve the effect of changing distribution, high activity and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0018] [Example 1] catalyst synthesis
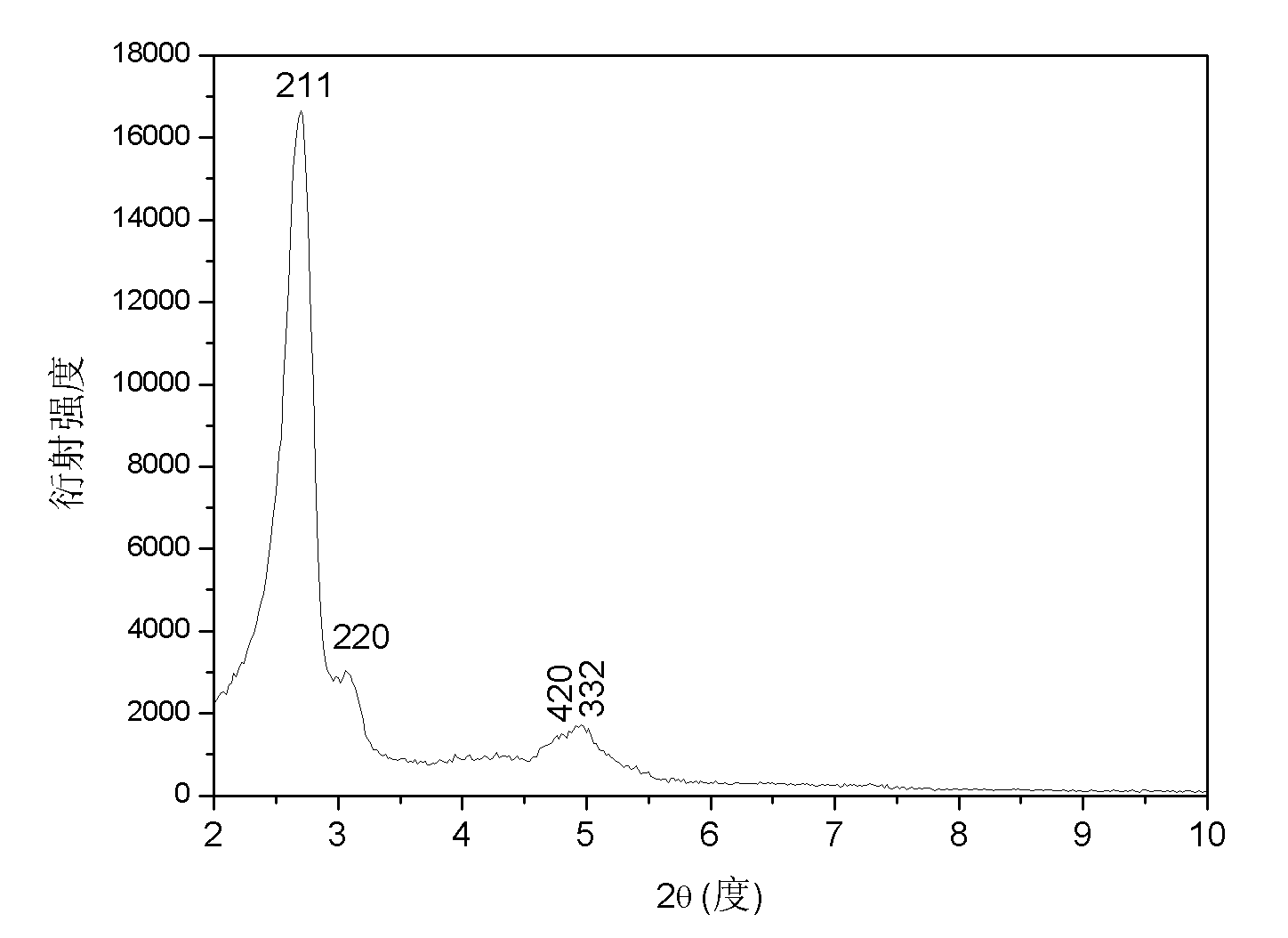
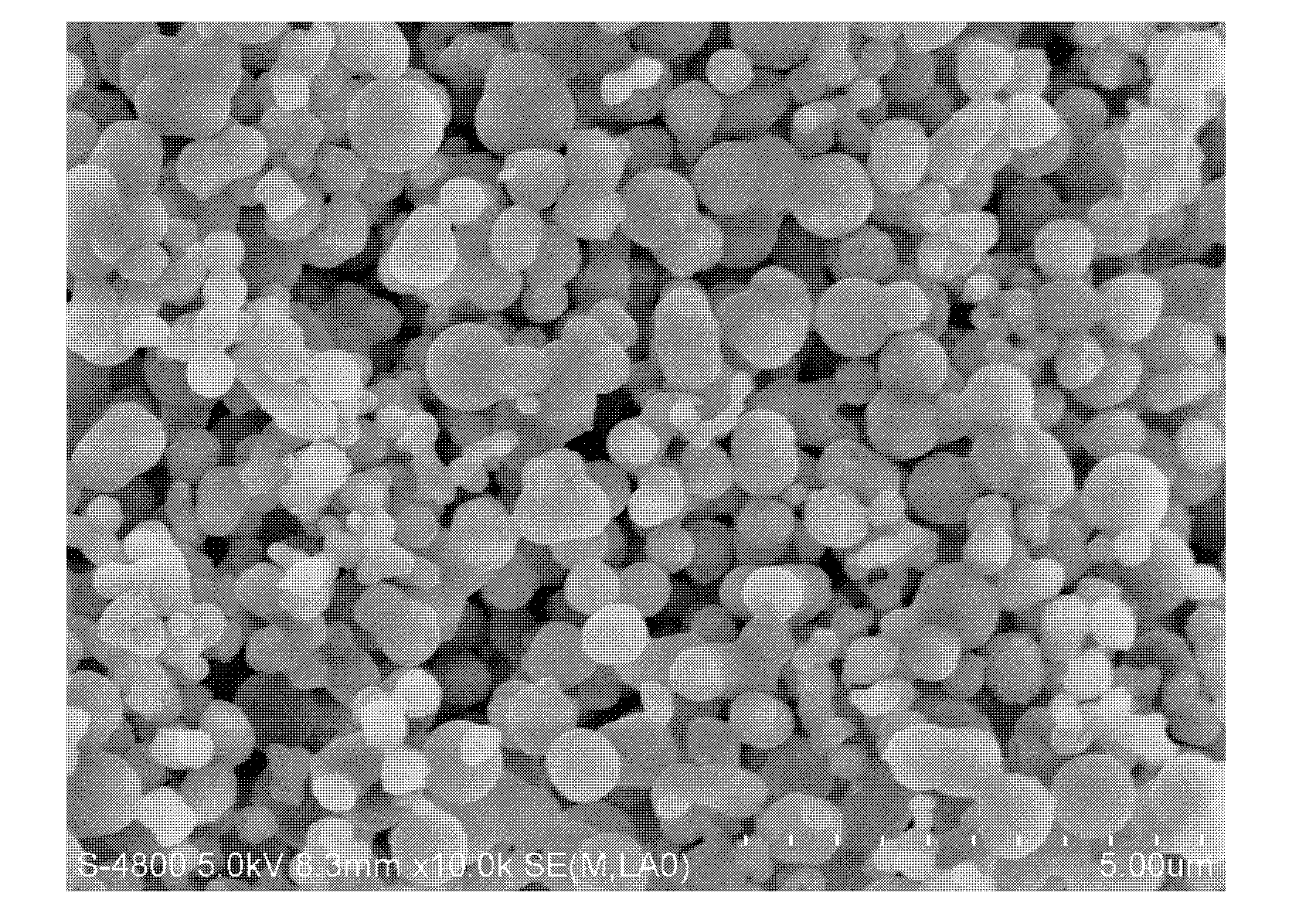
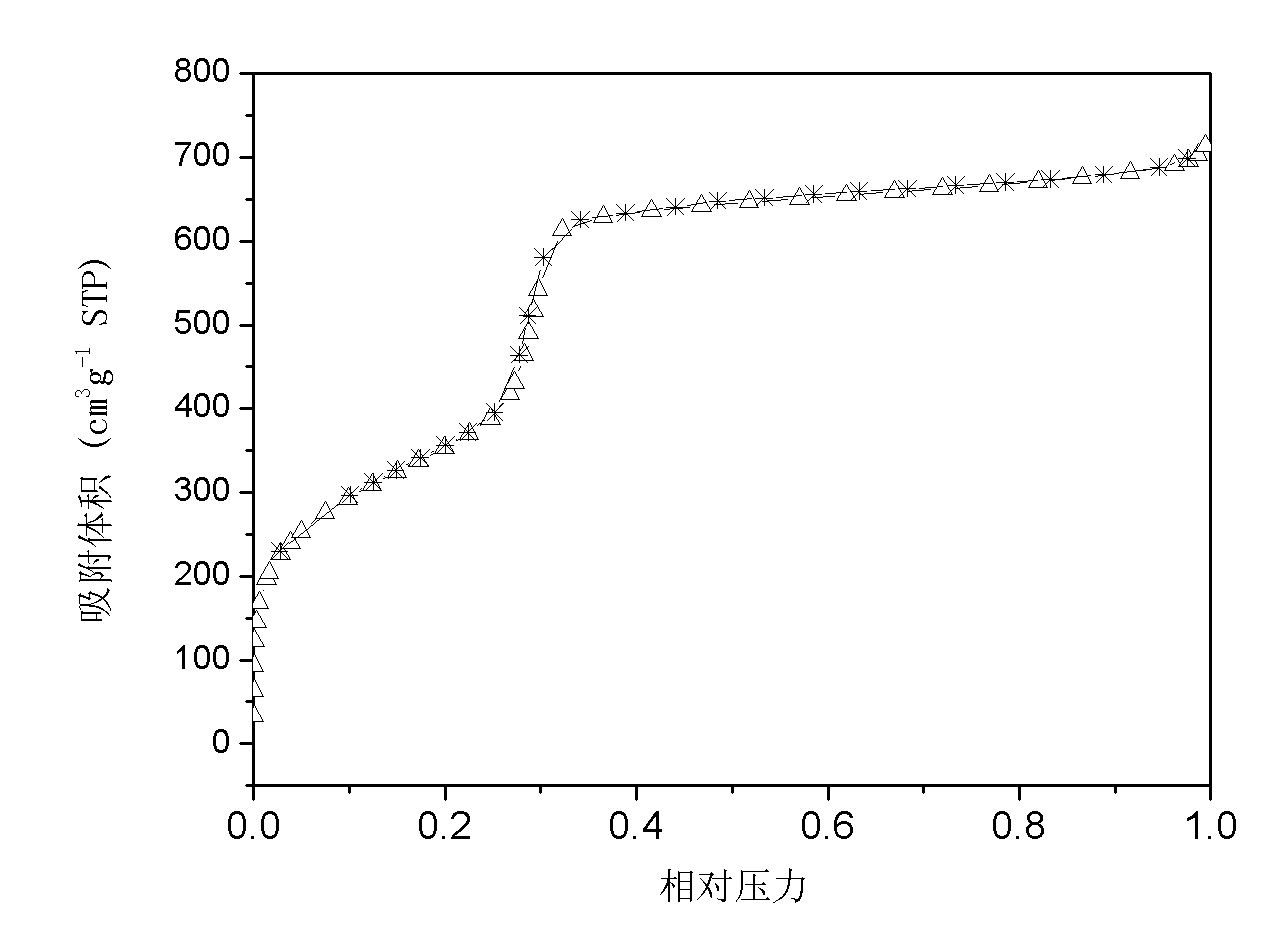
[0019] 1.0125g of cetyltrimethylammonium bromide was dissolved in 69.1ml of deionized water, 24.2ml of ethanol and 6.3ml of ammonia (NH 3 In the mixed solution whose mass concentration is 27%), it is recorded as solution A. At the same time, 0.0108 g of ammonium metavanadate was dissolved in 10 ml of deionized water, which was recorded as solution B. Slowly add B to A, followed by 2.1 ml tetraethylorthosilicate. After stirring for 1 h, the obtained gel was put into a reactor for hydrothermal reaction at 100° C. for 24 h. The solid obtained after centrifugation was washed, dried, and calcined in a muffle furnace at 550° C. for 6 h to remove the surfactant, and the obtained catalyst was designated as C1. The XRD, SEM and low-temperature nitrogen adsorption diagrams of sample C1 are detailed in Figure 1 ~ Figure 3 , it can be seen from the XRD pattern that this sample has a narrow peak at 2θ of 211 and three small peaks at 2θ of 3.0-5....
example 2
[0020] [Example 2] Synthesis of Catalyst
[0021] Dissolve 0.3355g nonadecyltrimethylammonium chloride in 69.1ml deionized water, 49.3ml propanol and 6.3ml ammonia water (NH 3 In the mixed solution whose mass concentration is 27%), it is recorded as solution A. At the same time, 0.216 g of ammonium metavanadate was dissolved in 10 ml of deionized water, which was recorded as solution B. Slowly add B to A, followed by 2.1 ml tetraethylorthosilicate. After stirring for 2 hours, the obtained gel was put into a reactor for hydrothermal reaction at 40° C. for 24 hours. The solid obtained after centrifugation was washed, dried, and calcined in a muffle furnace at 500° C. for 5 h to remove the surfactant, and the obtained catalyst was designated as C2. The catalyst has a spherical shape, and the diameter of the sphere is 0.1-1 μm.
example 3
[0022] [Example 3] Synthesis of Catalyst
[0023] 0.7013g of octaalkyltrimethylammonium bromide was dissolved in 62.1ml of deionized water, 21.9ml of ethylene glycol and 4.5ml of ammonia (NH 3 In the mixed solution whose mass concentration is 30%), it is recorded as solution A. At the same time, 0.0432 g of ammonium metavanadate was dissolved in 10 ml of deionized water, which was recorded as solution B. Slowly add B to A, followed by 2.1 ml tetraethylorthosilicate. After stirring for 3 hours, the obtained gel was put into a reactor for hydrothermal reaction at 80° C. for 12 hours. The solid obtained after centrifugation was washed, dried, and calcined in a muffle furnace at 600° C. for 7 h to remove the surfactant, and the obtained catalyst was designated as C3. The catalyst has a spherical shape, and the diameter of the sphere is 0.1-1 μm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com