Process for preparation of alpha-ketoglutaric acid
一种酮戊二酸、制备工艺的技术,应用在α-酮戊二酸的制备工艺领域,能够解决α-酮戊二酸制备方法复杂、生产成本增加、不能满足市场的需求等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
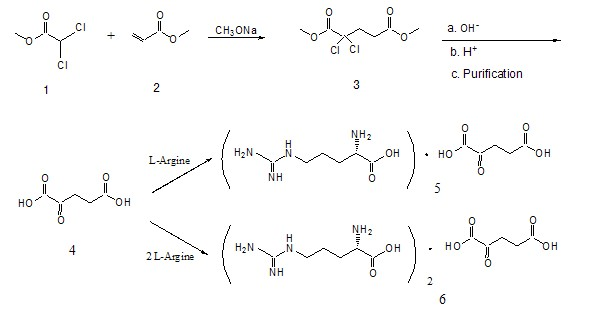
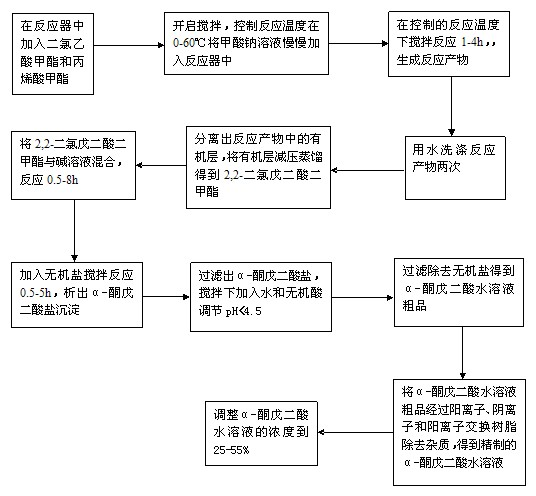
[0030] figure 1 and figure 2 It is a process step for preparing α-ketoglutaric acid in the present invention;
[0031] (a) Reaction of equimolar methyl dichloroacetate and methyl acrylate in the presence of sodium methoxide to obtain dimethyl 2,2-dichloroglutarate;
[0032] (b) Reaction of dimethyl 2,2-dichloroglutarate with alkali solution to obtain crude α-ketoglutarate aqueous solution;
[0033] (c) Purifying the crude α-ketoglutaric acid aqueous solution to obtain a refined α-ketoglutaric acid aqueous solution;
[0034] (d) adding water to adjust the concentration of the purified α-ketoglutarate aqueous solution;
[0035] The preparation of dimethyl 2,2-dichloroglutarate is further described as follows:
[0036] In the reactor, add equimolar methyl dichloroacetate and methyl acrylate, start stirring, and control the reaction temperature at 40°C to 50°C. Then slowly add a concentration of 30% sodium methoxide solution into the reactor, the weight of sodium methoxide i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com