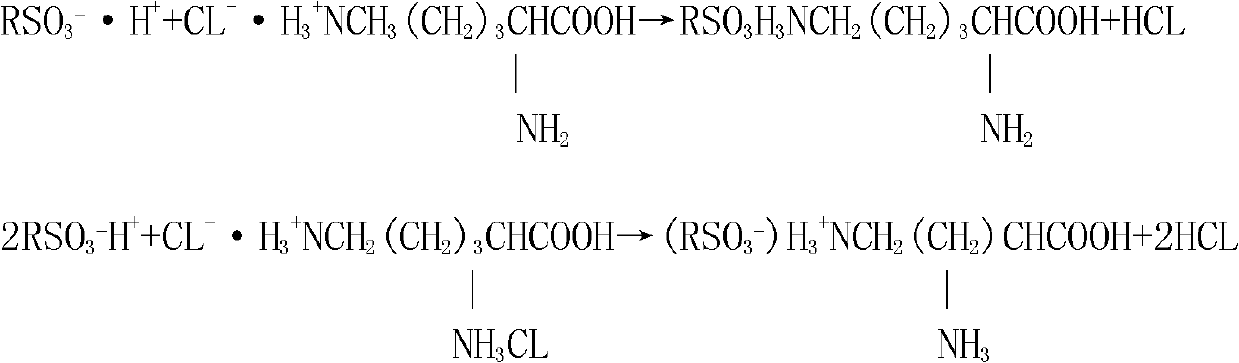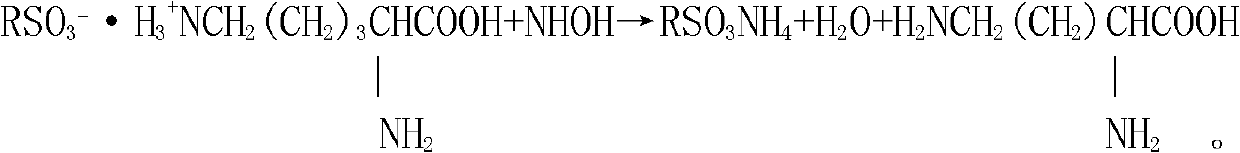Preparation method of L-lysine
A technology for lysine and lysine hydrochloride, which is applied in the field of preparing L-lysine using ion exchange resins, can solve the problems of large amount of compound reagents, increased production costs, long process routes, etc., and achieve product yield High efficiency, short reaction cycle and low raw material price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] (1) Add purified water (deionized water) to L-lysine hydrochloride, the weight ratio of L-lysine hydrochloride to purified water is 1:1, mix well and dissolve completely, and place it for use ;
[0026] (2) Column adsorption
[0027] Add the lysine obtained by step (1) into the ion exchange column equipped with cation exchange resin at a flow rate of 13 L / min until the resin cation exchange resin completely absorbs lysine, and the discharge of the column does not contain lysine , and then use purified water to wash through the resin in the exchange column until there is no Cl in the effluent of the column - (the pH of the effluent at this moment is 7);
[0028] (3) Elution
[0029] Then use 2mol / L NH 4 OH (ammonia water) elutes L-lysine, NH 4 OH flows through the ion-exchange column at a flow rate of 13L / min. When the pH value of the effluent of the ion-exchange column rises to 8.0, the effluent begins to react with ninhydrin, that is, L-lysine begins to flow out, ...
Embodiment 2
[0034] (1) Add purified water (deionized water) to L-lysine hydrochloride, the weight ratio of L-lysine hydrochloride to purified water is 0.5:1, mix well and dissolve completely, and place it for use ;
[0035] (2) Column adsorption
[0036] Add the solution obtained in step (1) into an ion exchange column equipped with a cation exchange resin at a flow rate of 12 L / min, until the resin cation exchange resin completely absorbs lysine, and the discharge of the column does not contain lysine , and then use purified water to wash through the resin in the exchange column until there is no Cl in the effluent of the column - (the pH of the effluent at this moment is 7);
[0037] (3) Elution
[0038] Then use 2mol / L NH 4 OH (ammonia water) elutes L-lysine, NH 4 OH flows through the ion-exchange column at a flow rate of 12L / min. When the pH value of the effluent of the ion-exchange column rises to 8.0, the effluent begins to react with ninhydrin, that is, L-lysine begins to flow...
Embodiment 3
[0043] (1) Add purified water (deionized water) to L-lysine hydrochloride, the weight ratio of L-lysine hydrochloride to purified water is 0.8:1, mix well and dissolve completely, and place it for use ;
[0044] (2) Column adsorption
[0045] Add the lysine obtained by step (1) into the ion exchange column equipped with cation exchange resin at a flow rate of 15 L / min until the resin cation exchange resin completely absorbs lysine, and the effluent of the column does not contain lysine , and then use purified water to wash through the resin in the exchange column until there is no Cl in the effluent of the column - (the pH of the effluent at this moment is 7);
[0046] (3) Elution
[0047] Then use 2mol / L NH 4 OH (ammonia water) elutes L-lysine, NH 4 OH flows through the ion-exchange column at a flow rate of 15L / min. When the pH value of the effluent of the ion-exchange column rises to 8.0, the effluent begins to react with ninhydrin, that is, L-lysine begins to flow out,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com