Aromatic acid compound and application
A technology of compounds and acids, applied in the field of medicine, can solve the problems of no methyl mandelate and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
[0026] Experimental example 1 Structural identification experiment of methyl mandelate
[0027] The structure of the mandelate methyl ester prepared in Examples 1-3 can be identified using this experimental example.
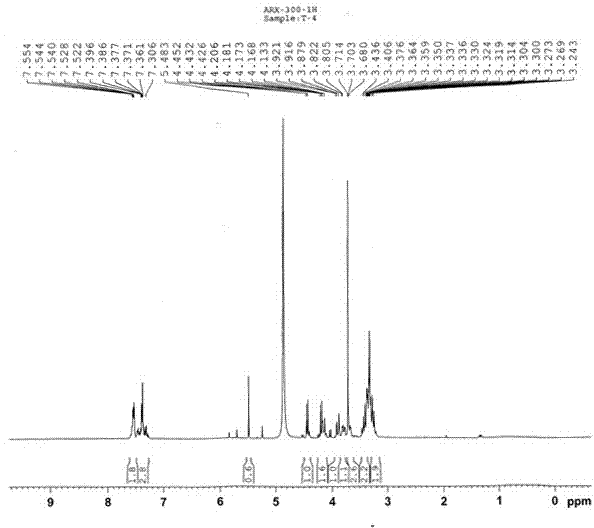
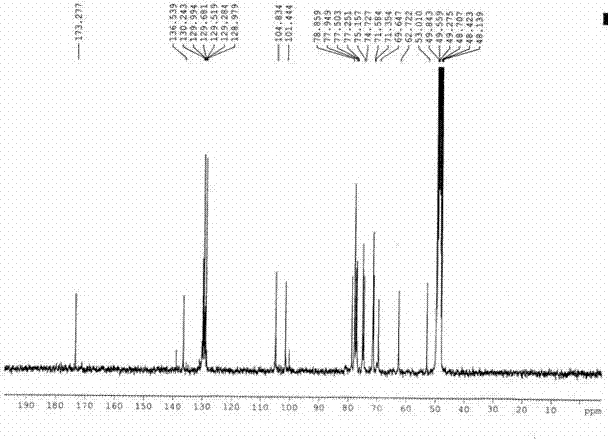
[0028] White powder (methanol), ESI-MS m / z : 513.05[M+Na] + , 529.05[M+K] + , according to which the molecular weight is determined to be 490.05, combined with 1 H-NMR and 13 C-NMR data, deduce that this compound molecular formula is C 21 h 30 o 13 . 1 H-NMR (300 MHz, CD 3 OD) spectrum, δ : 7.38(2H, m), 7.54 (3H, m) are characteristic hydrogen signals of monosubstituted benzene, δ :5.48(1H, s) is the benzyl hydrogen signal, δ : 4.28(1H, d, J = 7.6Hz), 4.45(1H, d, J = 7.6Hz) are the signals of 2 sugar anogroup protons, δ : 3.71(3H, s) is the methoxyl proton signal; 13 C-NMR (75Mz, CD 3 OD) spectrum, δ : 173.3 is the carboxyl carbon signal, and 104.8 and 101.4 are the sugar terminal carbon signals. Comparing the carbon and hydrogen dat...
experiment example 2
[0031] Experimental example 2 Scopolamine-induced memory loss in mice
[0032] (1) Experimental materials
[0033] 1. Experimental animals
[0034] ICR mice, male, weighing 18-22 g, were provided by the Experimental Animal Center of Shenyang Pharmaceutical University, license number: SCXK (Beijing) 2009-0004.
[0035]2. Drugs and reagents
[0036] Test drug: methyl mandelate, dissolved with 0.5% CMC-Na before administration, and made into a solution.
[0037] Scopolamine Hydrobromide Injection: Shanghai Hefeng Pharmaceutical Co., Ltd., production batch number: 080701, the dose for mice is 3 mg / kg; Naofukang, Hubei Huazhong Pharmaceutical Co., Ltd., batch number: 20091224.
[0038] Equipment: platform jumper, self-made.
[0039] (2) Experimental method
[0040] 1. Grouping and administration
[0041] The ICR mice were randomly divided into 5 groups according to body weight, namely blank control group, model group, positive control group, high-dose and low-dose groups...
Embodiment 1
[0051] Embodiment 1: Get 1kg of peach kernel medicinal material, pulverize, adopt 70% ethanol aqueous solution to reflux extract at 80 ℃ for 4h, extract is concentrated under reduced pressure to obtain concentrate; Butanol extraction, ethanol, 70% ethanol and 95% ethanol gradient elution; 30% ethanol eluate, concentrated under reduced pressure, dried, and silica gel column chromatography, using volume ratios of 9:1, 8:2, 7 :3, 6:4, 5:5 dichloromethane-methanol mixed solvent elution, and then separated and purified by preparative HPLC, with acetonitrile-water with a volume ratio of 95:5 as mobile phase elution to obtain mandelate A Esters 20mg.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com