Application of prenylated flavonoid compound
A kind of technology of isopentenyl flavonoid and compound, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
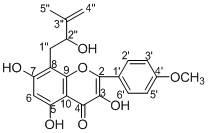
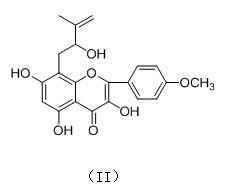
[0036] Example 1: The preparation of prenyl flavonoids --- dehydroicariin (I) and 2"-hydroxy-3"-ene-anhydroicariin (II), the specific steps are as follows:
[0037] Take dried Epimedium ( Epimedium brevicorum Maxim) whole herb 12 kg was pulverized, according to the mass / volume ratio of Epimedium: Acetone aqueous solution = 1:3, the concentration of 75% acetone aqueous solution was used to extract Epimedium three times at room temperature, and the amount of acetone water each time was 36 L, soaked for 24 hours, after combining the extracts for 3 times, distilled under reduced pressure until there is no acetone smell; according to the volume ratio of extract:petroleum ether=1:1, the extract was first extracted three times with petroleum ether at room temperature, The amount of petroleum ether used is 10 L each time. After the petroleum ether is recovered, the extract is obtained; according to the volume ratio of the extract: ethyl acetate = 1:1, the extract is extracted three ...
Embodiment 2
[0039] Embodiment 2: with Prenyl flavonoids are tablets prepared as active ingredients, and the tablet components and contents are as follows:
[0040] Active ingredient---prenyl flavonoid compound (I or II) 10mg
[0041] Lactose 156mg
[0042] Corn starch 55mg
[0044] Preparation method: mix the active ingredients, lactose, and cornstarch, moisten them evenly with water until they can be kneaded into agglomerates, press to make soft materials that are easy to disperse, dry at 60-80°C, sieve, add calcium stearate, and mix well Finally, it is compressed into tablets, each tablet weighs 225 mg, and the active ingredient content is 10 mg.
Embodiment 3
[0045] Embodiment 3: with Prenyl flavone compound is the spray prepared by the active ingredient, and its components and contents are as follows:
[0046] Active ingredient prenylflavone compound (I or II) 8mg
[0048] EDTA 0.5mg
[0049] Sodium Phosphate Buffer (PH6.5) 10mg
[0050] Add distilled water to 2ml
[0051] Preparation method: Add each solid component in distilled water in turn to dissolve completely, filter and sterilize through a microporous membrane, and bottle it.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com