Application of anti-angiohemophilia factor A3 zone bifunctional monoclonal antibody
A hemophilia factor and monoclonal antibody technology, applied in the field of medicine, can solve problems such as severe bleeding, hindering the clinical application of drugs, and severe bleeding side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1: animal grouping and design dosing regimen;
[0027] (1) Experimental animals
[0028] Yunnan Macaque (Suzhou Xishan Zhongke Experimental Animal Co., Ltd., experimental animal production license: SCXK (Su) 2007-0005; experimental animal use license: SYXK: (Su) 2006-0003. 2-3.5 years old, when the experiment started Weight 4.0-5.5Kg, 24, half male and half male.
[0029] (2) Dosing regimen
[0030] Macaque is negative control group (n=6) and administration group test (n=18), and administration group is divided into high dosage group (n=6), middle dosage group (n=6) and low dosage group (n=6) again. =6) Three groups, to study the effect of SZ-125 anti-arterial thrombosis drug in rhesus monkeys. The administration groups were divided into three groups of animals with high, middle and low doses, and the doses of macaques in each group were 0.6mg / Kg, 0.3mg / Kg and 0.1mg / Kg per kilogram of body weight respectively. The route of administration was aseptic intra...
Embodiment 2
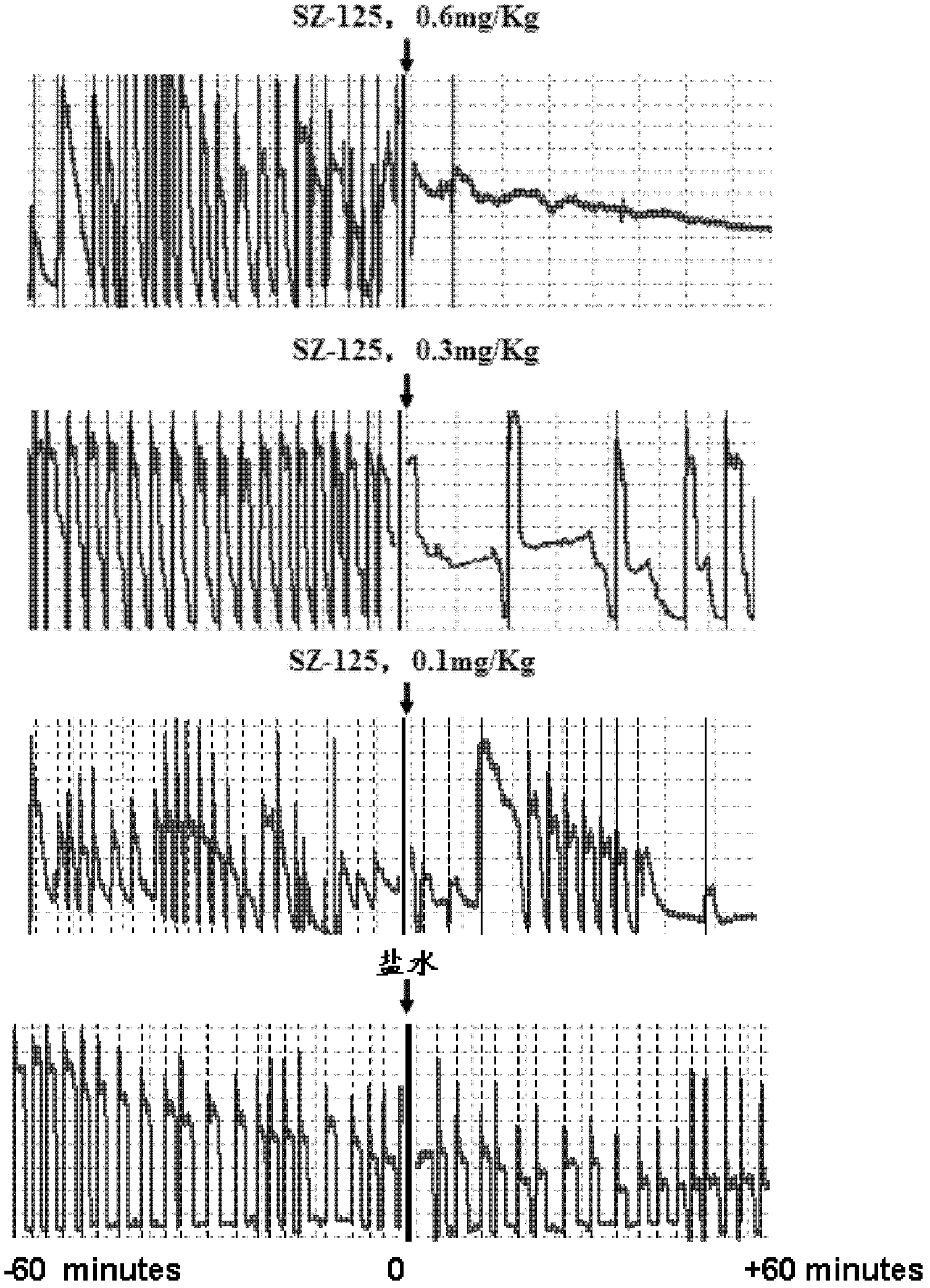
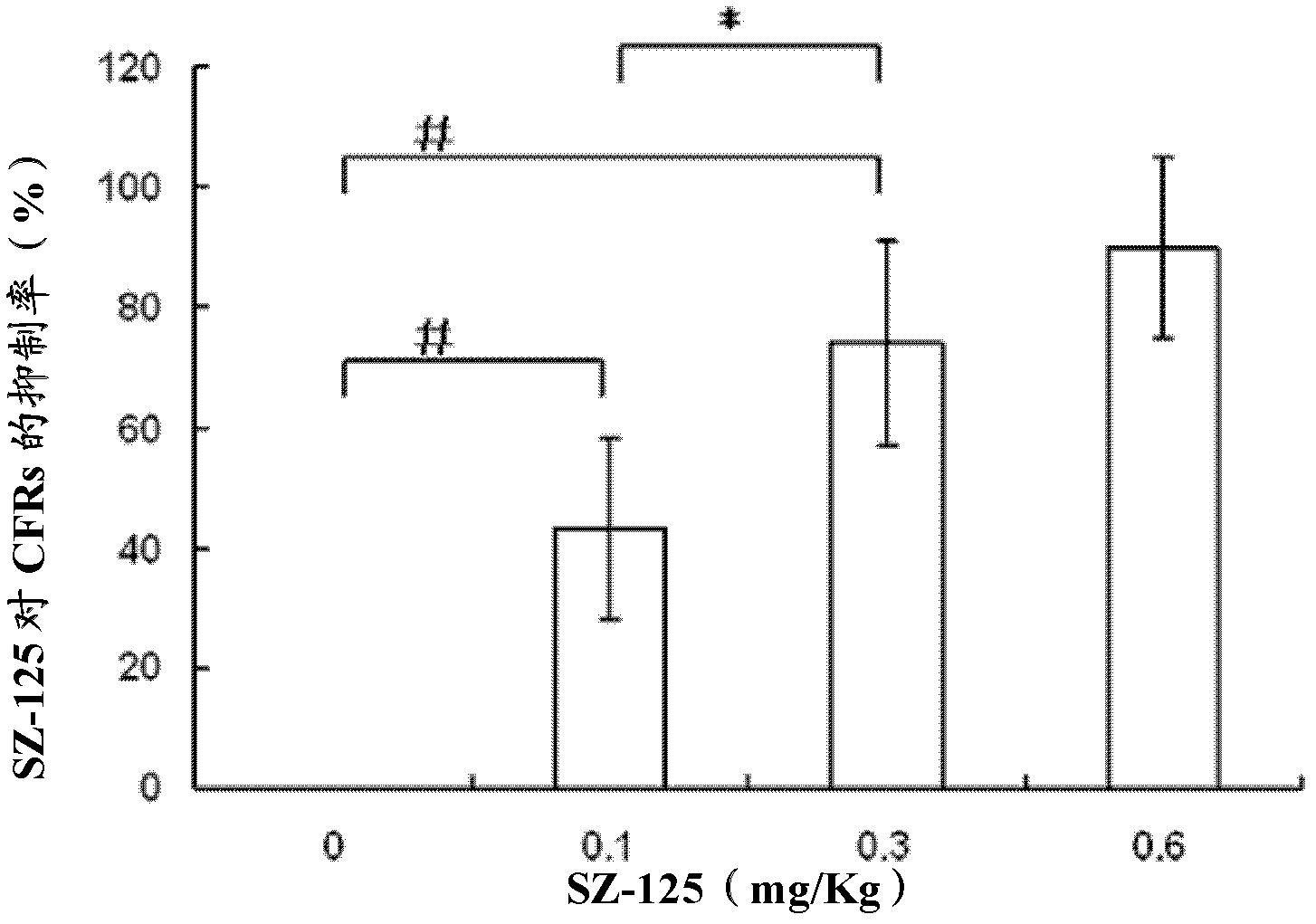
[0033] Example 2: Effects of Changes in SZ-125 Cynomolgus Monkey Periodic Reductions in Blood Flow (CFRs)
[0034] After the rhesus monkey was anesthetized by intravenous injection of 3% pentobarbital sodium (30 mg / kg), the temperature of the animal was maintained at 37° C. on a heating platform. The femoral artery was carefully separated with a length of about 5 cm, and a soft silicone C-type peripheral vascular ultrasound probe with a diameter of 0.5 cm was wrapped around the proximal blood vessel. The average flow velocity of the femoral artery was continuously recorded during the experiment. After the macaque was stabilized for 30 minutes, three arterial clips with spring devices were used to completely block the blood flow for 20 seconds, and the interval between each arterial clip was 2 mm to damage the vessel wall. Place a hard self-made C-ring with an appropriate diameter in the middle of the injured vessel, and insert a 3.0mm diameter balloon catheter (Cordis, USA, 3...
Embodiment 3
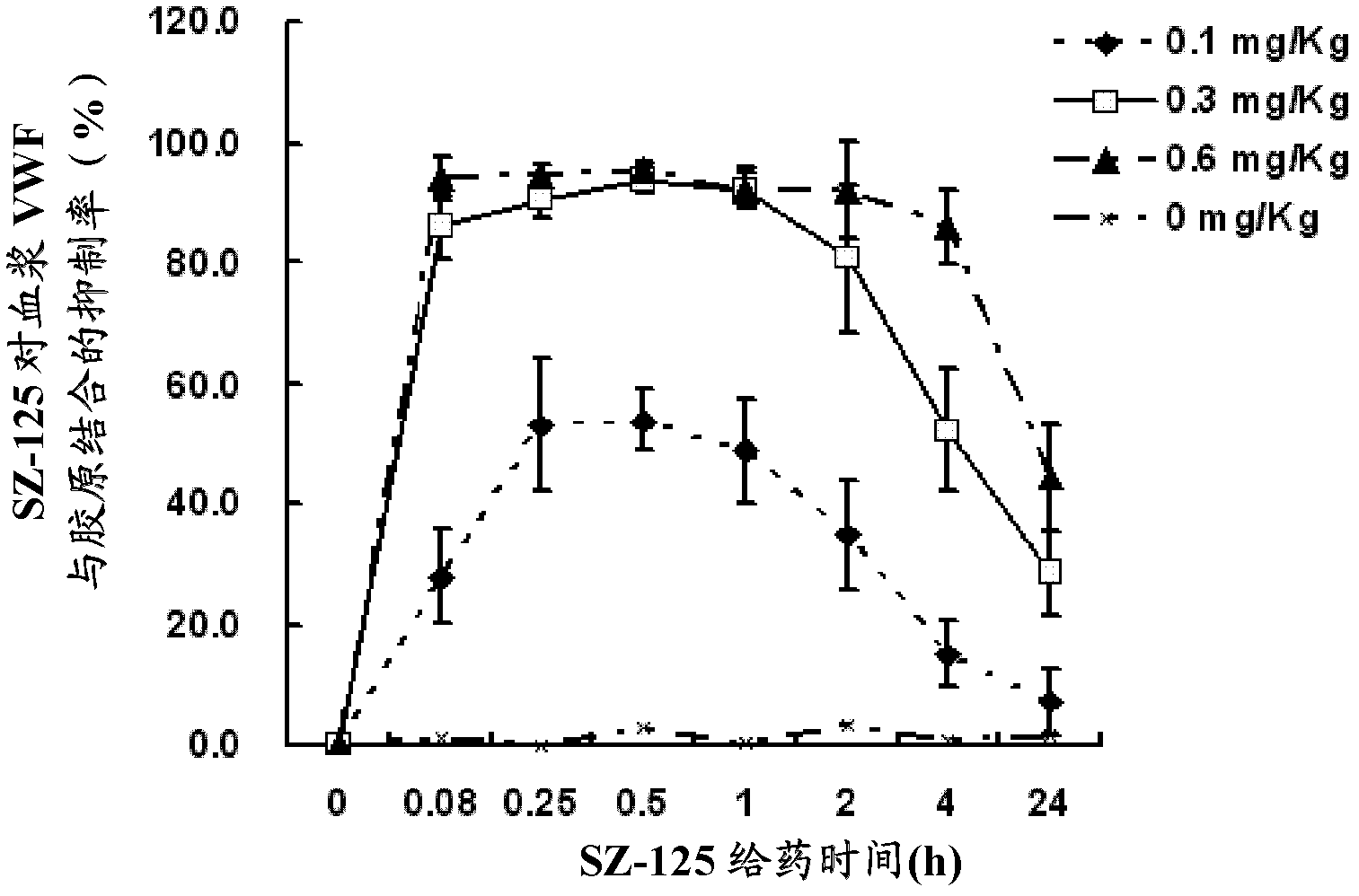
[0036] Example 3: SZ-125 inhibits the binding of macaque plasma VWF to collagen in vivo
[0037] Coating plate: Dissolve human placenta type III collagen (product of Sigma Company) in coating buffer, and coat it on a multi-well ELISA plate at 20 μg / mL, 100 μL / well, overnight at 4°C in a humid box; the next day, use PBS-0.05% After washing with Tween 3 times, block with 200 μL / well of blocking solution, and store overnight in a humid box at 4°C; wash 3 times with PBS-0.05% Tween the next day, and then store for later use or freeze at -20°C.
[0038] Detection: add 0.38% sodium citrate anticoagulated plasma at each time point of the macaque plasma to be tested at each time point obtained in Example 1 before and after administration to each well of the above-mentioned coated type III collagen microplate, blank Add 2% BSA-PBS solution to the control well, and incubate at 37°C for 2 h; wash with PBS-0.05% Tween for 3 times and add 1:4000 diluted horseradish peroxide-labeled rabbit ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com