Application for isobenzofuran type compounds in marine biofouling prevention and preparation method thereof
A marine biofouling and marine biological technology, applied to the preparation of isobenzofuran compounds, the application field of isobenzofuran compounds in the prevention of marine biofouling, can solve the destruction of marine ecological environment, natural non-toxic Complicated compound preparation process and other issues, to achieve the effect of high purity, strong inhibition of biofouling activity, and easy industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
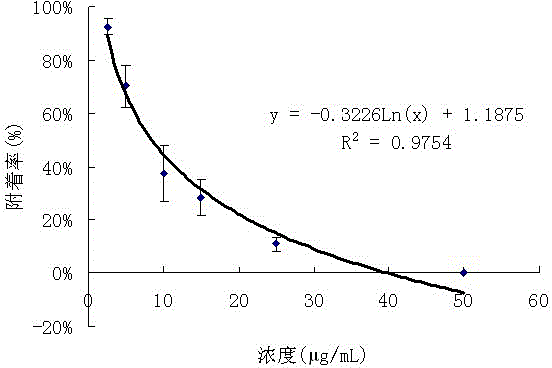
[0040] - Inhibitory activity of Cephalosporium AL031 fungal extract on the attachment of barnacle larvae.
[0041] Preparation of ethyl acetate extract of Cephalosporium AL031 fungal solid fermentation product: using rice, corn flour, bagasse, wheat bran as raw materials, mixing and sterilizing in a ratio of 10: 2: 3: 3 (W / W / W / W) ,cool down. Cephalosporium AL031 fungus was inoculated into the mixture, fermented at 28 °C for 6 days, the strain preservation number was CCTCC: M2010140; cold extraction was performed with industrial ethyl acetate for 3 days in the dark, extracted 3 times, the extract was filtered, reduced Press and concentrate to obtain ethyl acetate extract, which is stored in the dark.
[0042] An experimental model for inhibiting the attachment of barnacle Venus larvae was used (see scientific literature: Xu Y., He H. P., Qian P. Y, et al. Potent antifouling compounds produced by marine streptomyces. Bioresource Technology. 2010,101(4): 1331-1336) tested the a...
Embodiment 2
[0047] —— Preparation of 4,5,6-trihydroxy-7-methyl-isobenzofuran and 4,5,6-trihydroxy-7-methyl-isobenzofuranone.
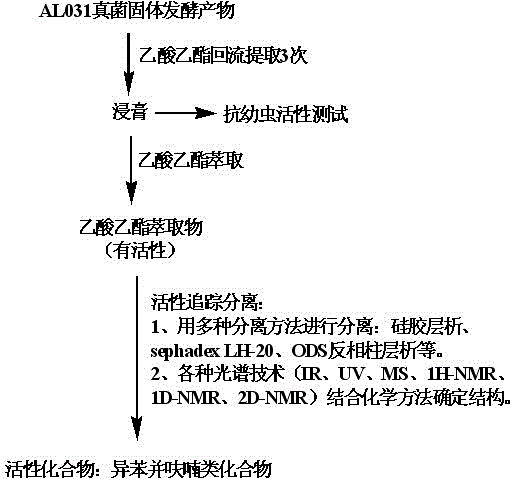
[0048] The isolation and identification process of the anti-biofouling active components in the solid fermentation products of Cephalosporium AL031 fungi is as follows: figure 1 shown.
[0049] Using rice, corn flour, bagasse, and wheat bran as raw materials, mix sterilization and cooling in a ratio of 10: 2: 3: 3 (W / W / W / W); inoculate the fungal strain named Cephalosporium AL031 In the mixed medium, fermented at 28 °C for 6 days, the strain preservation number is CCTCC: M2010140; the solid fermentation product was extracted with redistilled industrial ethyl acetate in the dark for 3 days, extracted 3 times, combined with the extract, Filter and concentrate the extract under reduced pressure to obtain ethyl acetate extract (40 g), disperse the above-mentioned re-distilled industrial ethyl acetate extract in water, use 10 g of re-distilled industrial ethyl acetate...
Embodiment 3
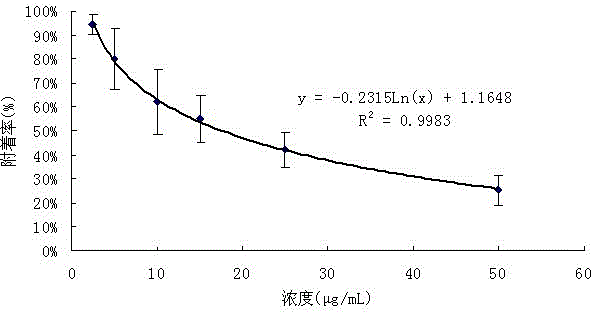
[0056] ——Determination of anti-barnacle larva attachment activity of compounds 4,5,6-trihydroxy-7-methyl-isobenzofuran and 4,5,6-trihydroxy-7-methyl-isobenzofuranone.
[0057] Using the experimental model of inhibiting the attachment of barnacle Venus larvae (see scientific literature: Xu Y., He H. P., Qian P. Y., et al. Potent antifouling compounds produced by marine streptomyces. Bioresource Technology. 2010,101(4):1331- 1336), to test the anti-barnacle larva attachment activity of compounds. Barnacle barnacle adults (Darwin) were collected from the intertidal zone of Hong Kong (22°19'N, 114°16'E). Put 8 L of filtered seawater in a 12 L polystyrene plastic culture container, then put the adult barnacle barnacles into the container, let it release the larvae, and collect the larvae after 2.5 h. The larvae at this stage are called nonodes Larvae (nauplius), incapable of attachment. The nauplii were placed in a container filled with 8 L of filtered seawater (the pore size of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com