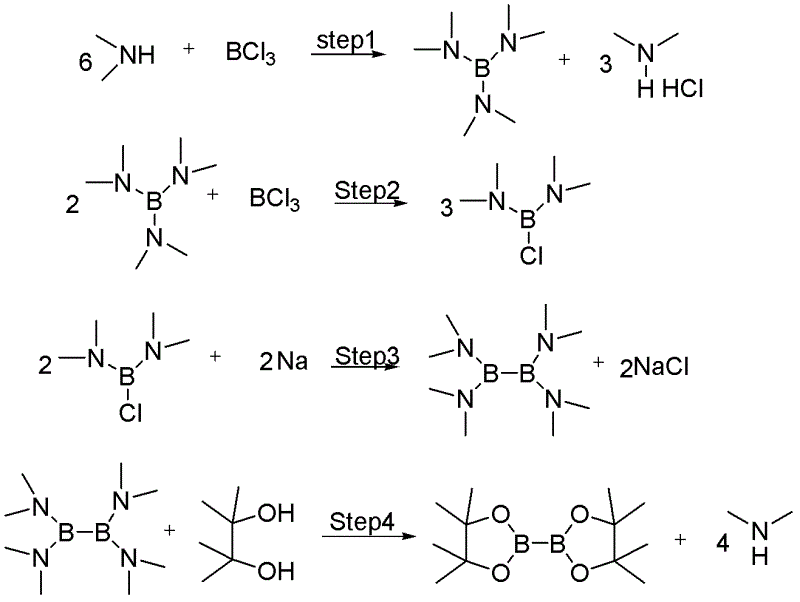
Method for synthesizing bis(pinacolato)diboron
A technology of biboronic acid pinacol ester and chloroborane, which is applied in the field of organic synthesis and preparation chemistry, can solve the problems of unsuitability for large-scale production, high price of boron tribromide, unsafe operation, etc., and achieve stable conversion rate and purity , Facilitate solvent recovery and reduce loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1: a kind of synthetic method of biboronic acid pinacol ester is characterized in that concrete steps are as follows:
[0027] (1) Triamino substitution: Add 200kg (444.4mol, 1.0eq) of the main raw material 10% dimethylamine in n-hexane (14mL / g) to a 500L reactor, and add 10% trichloride dropwise at -20±2°C Boron chloride n-hexane (7mL / g) solution 104.2kg (88.9mol, 0.2eq), after dropping, the system was warmed up to 5 ± 2°C, and kept warm until the end of the NMR detection reaction; after the reaction, add 26kg of n-hexane ( 2mL / g), stirring, and centrifuging, the obtained filtrate is 306kg (9.5kg, 66.6mol) of n-hexane solution of tris(dimethylamino)borane, the yield is 90%, and the next step is to be cast;
[0028] (2) Diamino substitution: 306 kg (9.5 kg, 66.6 mol) of the n-hexane solution of tris(dimethylamino) borane prepared in step (1) was added to a 500L reactor, and added dropwise at -20±2°C 10% boron trichloride in n-hexane (9mL / g) solution 62.5kg (...
Embodiment 2
[0032] (1) Triamino substitution: Add 200kg (1111.1mol, 1.0eq) of 25% dimethylamine in n-heptane (4mL / g) as the main raw material to a 500L reactor, and add 25% Tris Boron chloride in n-heptane (2mL / g) solution 88.6kg (188.9mol, 0.17eq), after dropping, the system was heated up to 15±2°C, and kept warm until the reaction was detected by NMR; after the reaction, add n-heptane to the system Alkane 34kg (1mL / g), stirred, centrifuged, the obtained filtrate is 274kg (26.5kg, 185.2mol) of n-heptane solution of tris(dimethylamino)borane, the yield is 100%, and the next step is to be cast;
[0033] (2) Diamino substitution: Add 274kg (26.5kg, 185.2mol) of the n-heptane solution of tris(dimethylamino)borane prepared in step (1) to a 500L reactor, and drop it at -5±2°C Add 43.4kg (92.6mol, 0.5eq) of n-heptane (2mL / g) solution of 25% boron trichloride, after dropping, the system is warmed up to 15±2°C, and kept warm until the NMR detection reaction ends; Diatomaceous earth 13.3kg (0.5g / ...
Embodiment 3
[0037] (1) Triamino substitution: 200kg (1666.7mol, 1.0eq) solution of 45% dimethylamine in cyclohexane (2mL / g) as the main raw material was added to a 1000L reactor, and 45% trichloride was added dropwise at 0±2°C Boron compound cyclohexane (1mL / g) solution 106.5kg (500.0mol, 0.3eq), after dropping, the temperature of the system was raised to 25±2°C, and the temperature was kept until the end of the NMR detection reaction; after the reaction was completed, cyclohexane was added to the system 273kg (5mL / g), stirred and centrifuged, the obtained filtrate is 553.3kg (37.7kg, 263.9mol) of cyclohexane solution of tris(dimethylamino)borane, the yield is 95%, and the next step is to be cast;
[0038] (2) Diamino substitution: Add 553.3kg (37.7kg, 263.9mol) of the cyclohexane solution of tris(dimethylamino)borane prepared in step (1) to a 1000L reactor, and drop it at 0±2°C Add 68.7kg (263.9mol, 1.0eq) of 45% boron trichloride cyclohexane (1mL / g) solution, after dropping, the system ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com