Preparation method of phytostanol ester by taking ionic liquid as catalyst
A technology of phytostanol esters and phytostanols, which is applied in the field of preparing fatty acid or hydroxy acid phytostanol esters, can solve the problems of limited application range, poor fat solubility and water solubility of phytostanols, and achieve The effect of convenient preparation, green synthesis route, and expanded application range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
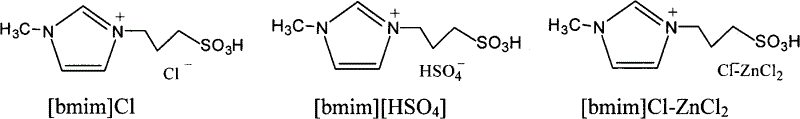
[0020] Add 2.08g of phytostanol and 6.00g of lauric acid into a reaction test tube with a branch, blow nitrogen, dissolve completely at 130°C under magnetic stirring conditions, add 0.13g of [bmim]Cl-ZnCl 2 , and controlled the temperature of 130 ° C under the condition of esterification for 6 hours, separated and purified to obtain lauric acid phytostanol ester.
Embodiment 2
[0022] Add 2.08g of phytostanol and 4.05g of myristic acid into a reaction test tube with a branch, blow nitrogen, dissolve completely at 135°C under magnetic stirring conditions, add 0.08g of [bmim][HSO 4 ], esterification reaction at a controlled temperature of 135 ° C for 5 h, separation and purification of phytostanol myristate.
Embodiment 3
[0024] Add 1.04g phytostanol and 3.50g linoleic acid into a reaction test tube with a branch, blow nitrogen, dissolve completely at 160°C under magnetic stirring, add 0.05g [bmim]Cl, control the temperature at 160°C The reaction was carried out for 4 hours, and the linoleic acid phytostanol ester was obtained by separation and purification.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com