Extraction method of tocopherol intermediate
A technology of tocopherol and intermediates, which is applied in the field of efficient extraction of tocopherol intermediates, can solve problems such as removal, achieve stable reaction process, stabilize production technology, and avoid oxidative deterioration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
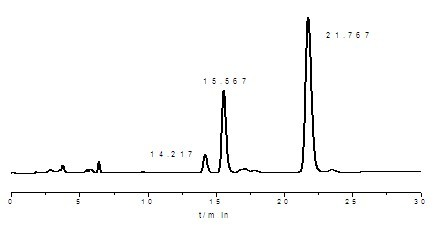
[0037] All instruments and equipment are laboratory-scale. D-α-tocopherol accounts for 7.67% in the amine methylation product of tocopherol concentrated liquid, D-β, γ-tocopherol intermediates account for 61.06%, and D-δ-tocopherol intermediates body accounted for 26.97%.
[0038] Take 20g of tocopherol concentrated liquid amine-methylated product (about 30mL) into the reactor, add 50mL of aqueous solution with 90% acetic acid content, then add 60mL of n-heptane reagent, control the temperature at about 30°C, and stir for 0.5h; Then stand for stratification, the lower layer is the water phase of acetic acid, which is transferred to the desolventizer, heated to 120°C and distilled under reduced pressure to evaporate the acetic acid to obtain the non-D-α-tocopherol intermediate salt; finally add 10.7mL of mass The dimethylamine aqueous solution with a fraction of 40% was stirred at 35°C for 20 minutes, then the stirring was stopped, and 50 mL of deionized water was added to wash...
Embodiment 2
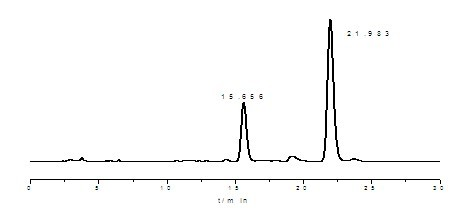
[0041]Take 20g of tocopherol concentrated liquid amine-methylated product (about 30mL) into the reactor, add 50mL of aqueous solution with 80% acetic acid content, then add 60mL of petroleum ether (60-90°C) reagent, control the temperature at about 35°C, Stir and react for 1.0 h; then stand to separate and separate, the lower layer is the aqueous phase of acetic acid, transfer it to the desolventizer, heat up to 120°C and distill the acetic acid under reduced pressure to obtain the non-D-α-tocopherol intermediate salt; Finally, add 10.7mL of dimethylamine aqueous solution with a mass fraction of 40%, stir at 35°C for 20min, stop stirring, add 50mL of deionized water to wash the feed solution and let it stand for stratification. The upper oil phase is the obtained non-α-tocopherol intermediate .
[0042] Take 1-2 drops of the product and weigh it and prepare 25mL diluted solution with absolute ethanol. Through reverse-phase high-performance liquid chromatography HPLC, using met...
Embodiment 3
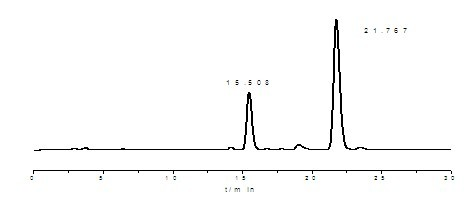
[0044] Take 20g of tocopherol concentrated liquid amine-methylated product (about 30mL) into the reactor, add 60mL of aqueous solution with 80% propionic acid content, then add 60mL of n-hexane reagent, control the temperature at about 30°C, and stir for 1.0h; Then stand for stratification, the lower layer is the water phase of propionic acid, which is transferred to the desolventizer, heated up to 140 °C and distilled under reduced pressure to evaporate the propionic acid to obtain the non-D-α-tocopherol intermediate salt; finally add 10.7 40% aqueous solution of dimethylamine in mL was stirred at 35°C for 20 minutes, then the stirring was stopped, 50 mL of deionized water was added to wash the feed solution, and the mixture was allowed to stand for stratification. The upper oil phase was the obtained non-α-tocopherol intermediate.
[0045] Take 1-2 drops of the product and weigh it and prepare 25mL diluted solution with absolute ethanol. Through reverse-phase high-performance...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com