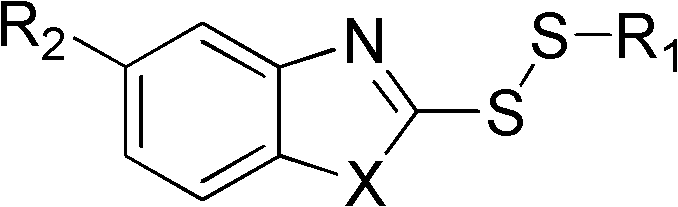
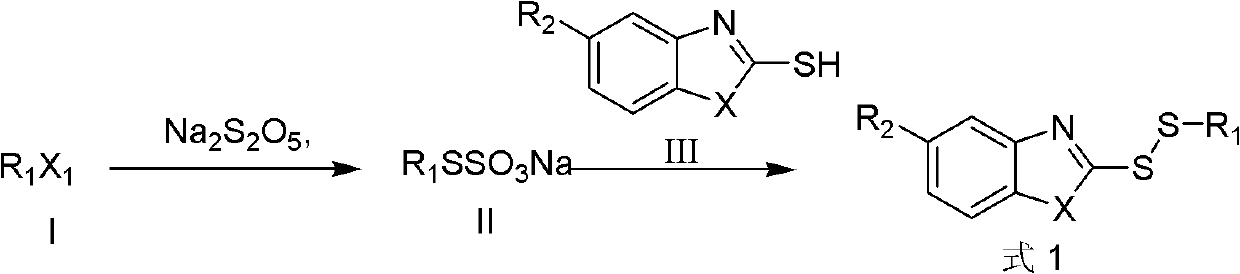
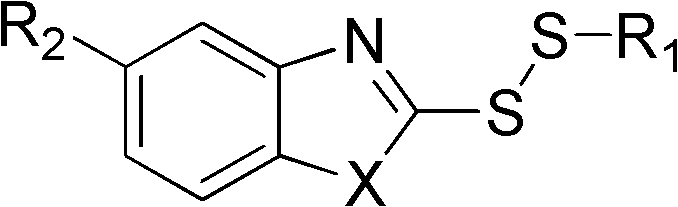
Disulfide compound as well as preparation method and application thereof
A disulfide and compound technology, applied in the field of new disulfide compounds and their synthesis, can solve the problems of human and environmental hazards, alkyl thiol odor, etc., and achieve low production cost, simple operation, and good anti-tumor activity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] The preparation of embodiment 1, sodium ethyl thiosulfate:
[0036] Mix 10mmol ethyl iodide, 2.48g sodium thiosulfate pentahydrate with 25mL 75% ethanol-water solution, heat and reflux for 2 hours, after detection the sodium thiosulfate completely disappears, lower the temperature, and use it directly for the next reaction.
Embodiment 2
[0037] Embodiment 2, the preparation of ethyl-(2-benzoxazole) disulfide (1a)
[0038] Take 1.51g of 2-mercaptobenzoxazole, mix it with 10mL of water, add 30% KOH dropwise until the solid is completely dissolved, pour it into the above-mentioned sodium ethyl thiosulfate solution, continue to stir at room temperature for 30min, and evaporate under reduced pressure. Part of the ethanol was extracted with ether, dried over magnesium sulfate, concentrated by filtration, and the residue was subjected to silica gel column chromatography (petroleum ether and ether as eluents) to obtain a colorless oil. Yield 75%.
[0039] 1 H-NMR (CDCl 3 ), δ (ppm): 1.43 (t, 3H, CH 3 ), 3.02(q, 2H, CH 2 ), 7.30(m, 2H, ArH), 7.50(m, 1H, ArH), 7.68(m, H, ArH); ESI-Mass: 211.9(M+1) + .
Embodiment 3
[0040] Embodiment 3, the preparation of ethyl-(2-benzothiazole) disulfide (1b)
[0041] Referring to Example 2, just change 2-mercaptobenzoxazole into 2-mercaptobenzothiazole. The product is a colorless oil with a yield of 70%; 1 H-NMR (CDCl 3 ), δ (ppm): 1.41 (t, 3H, CH 3 ), 2.98(q, 2H, CH 2 ), 7.30 (m, 2H, ArH), 7.42 (m, H, ArH), 7.79 (dd, H, ArH), 7.86 (dd, H, ArH); ESI-Mass: 227.9 (M+1) + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com