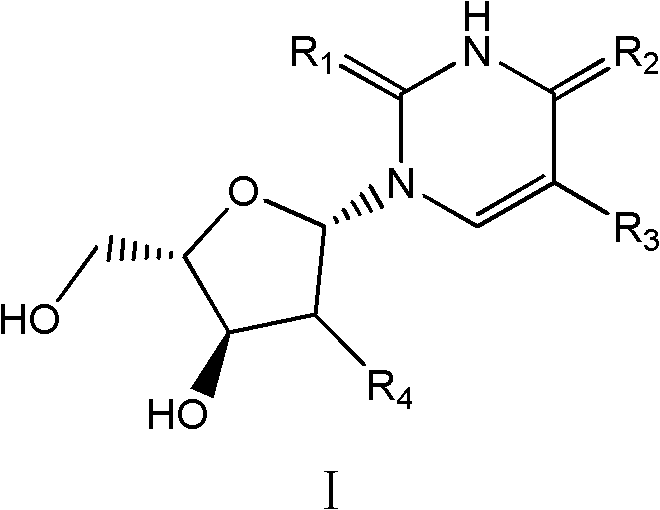
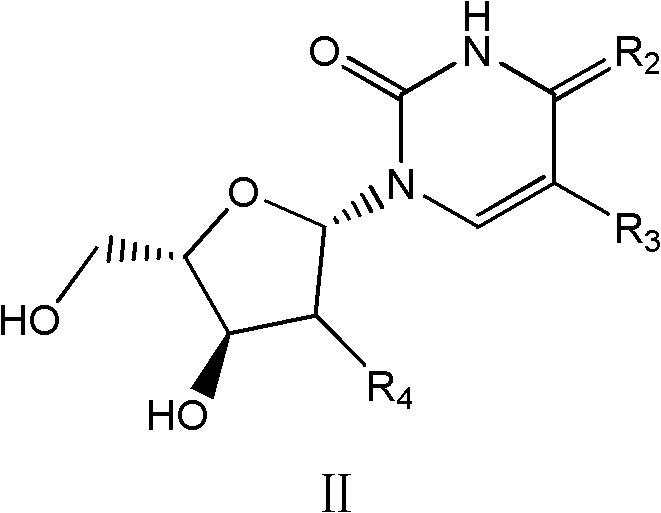
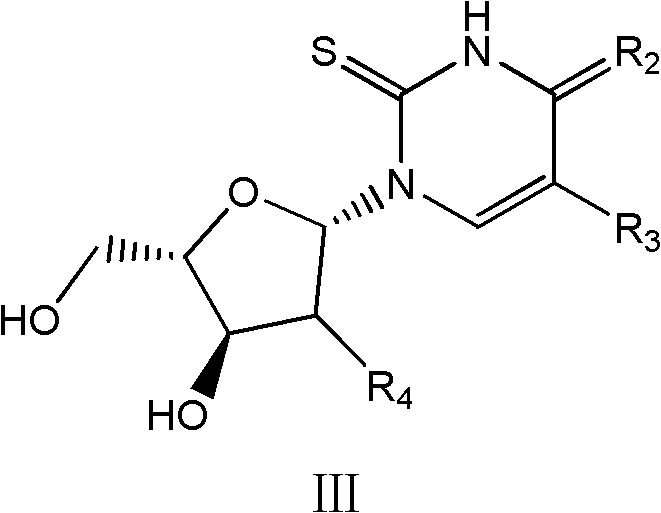
Beta-L-2'-desoxy-thymin-nucleoside derivative, preparation method and purposes thereof
A technology of thymidine derivatives and thymidine, which is applied in the field of chemical medicine and can solve the problems of easy drug resistance of viruses, nephrotoxicity, and easy rebound after drug withdrawal
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 52
[0250] Example 52, 4-dithio-2'-deoxy-L-thymidine
[0251]
[0252] a. Synthesis of Compound 19
[0253] Take 7.8g of compound 14, add 200ml of anhydrous pyridine, stir to dissolve, add 7.5ml of benzoyl chloride, heat to 50°C and react for 10-20 hours, after the reaction is detected by TLC, concentrate to about 30ml, add crushed ice while stirring, A precipitate was formed, filtered, dried under pressure, and recrystallized from ethanol to obtain 12.5 g of compound 19.
[0254] b. Synthesis of Compound 20
[0255] Take 9.4g of compound 19, add 200ml of dioxane, stir to dissolve, add 14g of Lawson's reagent, reflux reaction, TLC monitors the reaction progress, after the reaction is completed, concentrate to dryness, add water, extract with chloroform, the water layer is concentrated to dryness, and the column Chromatography yielded 8.5 g of compound 20.
[0256] c. Synthesis of Compound 21
[0257] Take 7g of compound 20, add 200ml of methanol, stir to dissolve, add 1.8g ...
Embodiment 72
[0266] Example 72, 4-diselenium-2'-deoxy-L-thymidine
[0267]
[0268] a. Synthesis of Compound 25
[0269] 35ml POCl 3 Add dropwise to containing 100g1,2, in the anhydrous acetonitrile suspension (1000ml) of 4-triazole, N 2 protection, stirred at 0°C for 10 min; then 200ml of anhydrous Et 3 N was added dropwise to the above mixed solution, and stirred at 0°C for 35 min; finally, acetonitrile solution (500 ml) in which 11 g of compound 22 was dissolved was added, and the reaction was stirred at room temperature. TLC detected that the raw material point disappeared, and about 200 ml of saturated NaHCO was added. 3 , and then extracted with ethyl acetate, washed with water, dried and concentrated to obtain 10.6 g of compound 25.
[0270] b. Synthesis of Compound 26
[0271] 3gNaBH 4 Add to containing 6ml (NCCH 2 CH 2 Se) 2 and 200ml of ethanol in a round bottom flask, ice bath, N 2 A solution of 10 g of compound 25 in THF (100 ml) was added under protection, and the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com