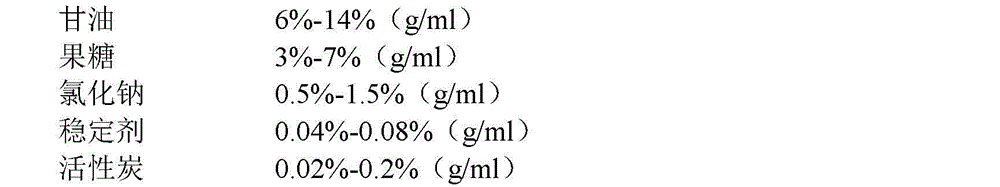Mannitol sodium chloride injection and preparation method thereof
A sodium chloride injection, injection technology, applied in the direction of medical formula, blood disease, extracellular fluid disease, etc., to achieve the effect of solving the problem of easily exceeding the standard
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] A kind of glycerol fructose sodium chloride injection, each raw material of this injection, adjuvant consists of:
[0045]
[0046] 1mol / L hydrochloric acid to adjust the pH value to 4.3;
[0047] Add water for injection to 1000ml.
[0048] The specific steps are:
[0049] (1) Weigh each raw material and auxiliary material in proportion, and set aside;
[0050] (2) Add glycerin and sodium chloride to 20% of the total amount of water for injection to dissolve;
[0051] (3) After adding sodium bisulfite to the above solution to dissolve, add fructose to dissolve;
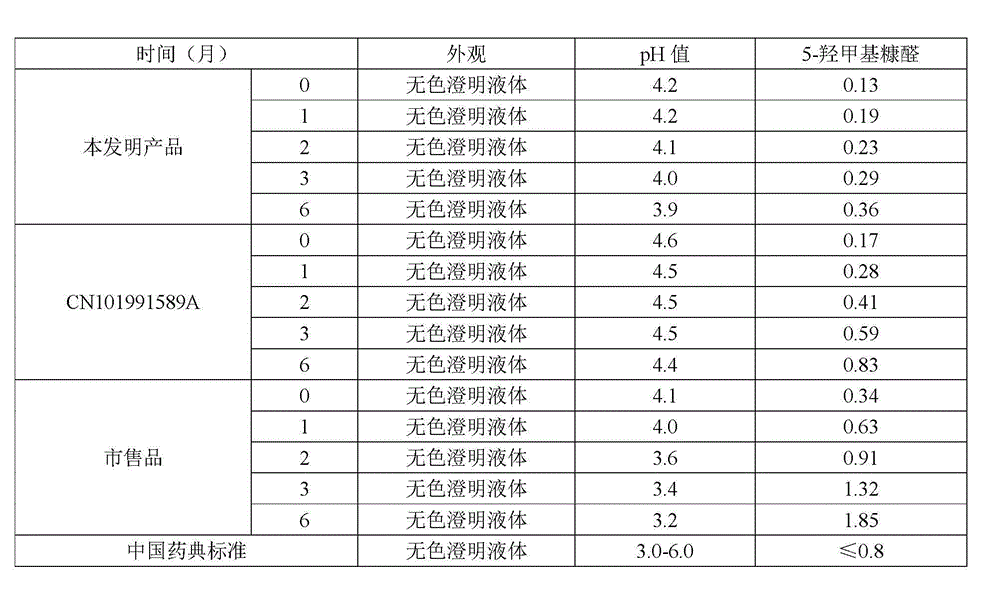
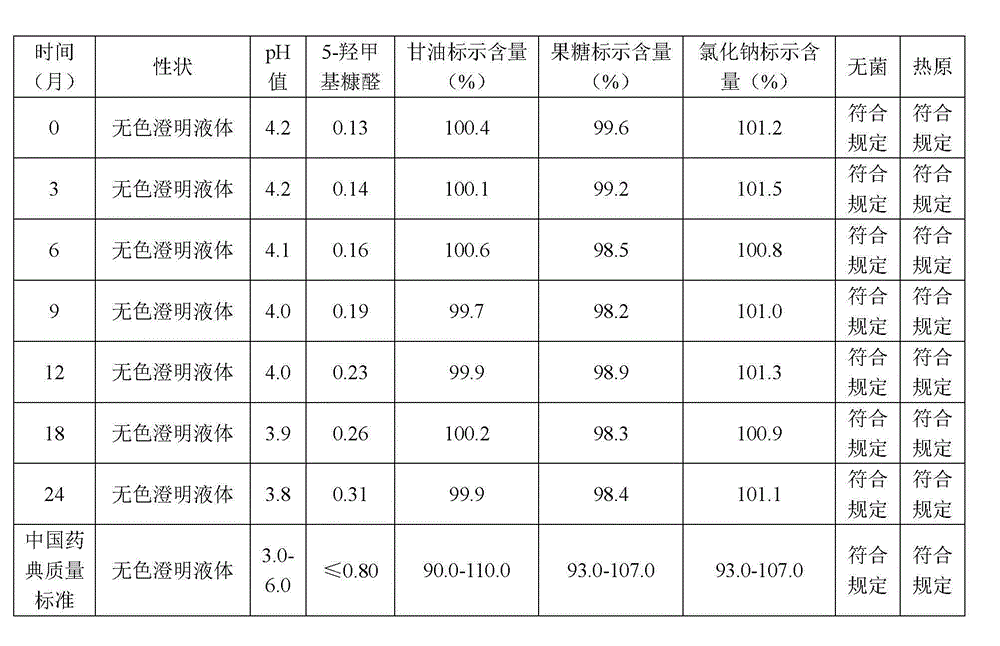
[0052] (4) Add 1mol / L hydrochloric acid to the above solution to adjust the pH value to 4.3, add water for injection to the full amount, add activated carbon, stir and mix well, and measure the marked content of glycerin, sodium chloride, and fructose at 95.0-105.0%;
[0053] (5) Coarsely filter and finely filter the above medicinal solution, fill, seal, sterilize with damp heat at 121°C for 8 minutes, i...
Embodiment 2
[0055] A kind of glycerol fructose sodium chloride injection, each raw material of this injection, adjuvant consists of:
[0056]
[0057] 1mol / L hydrochloric acid to adjust the pH value to 4.6;
[0058] Add water for injection to 1000ml.
[0059] The specific steps are:
[0060](1) Weigh each raw material and auxiliary material in proportion, and set aside;
[0061] (2) Add glycerin and sodium chloride to 50% of the total amount of water for injection to dissolve;
[0062] (3) After adding sodium sulfite to the above solution to dissolve, add fructose to dissolve;
[0063] (4) Add 1mol / L hydrochloric acid to the above solution to adjust the pH value to 4.6, add water for injection to the full amount, add activated carbon, stir and mix well, and measure the marked content of glycerin, sodium chloride, and fructose at 95.0-105.0%;
[0064] (5) Coarsely filter and finely filter the above medicinal solution, fill, seal, sterilize with damp heat at 121°C for 8 minutes, insp...
Embodiment 3
[0066] A kind of glycerol fructose sodium chloride injection, each raw material of this injection, adjuvant consists of:
[0067]
[0068] 1mol / L sulfurous acid to adjust the pH value to 4.2;
[0069] Add water for injection to 1000ml.
[0070] The specific steps are:
[0071] (1) Weigh each raw material and auxiliary material in proportion, and set aside;
[0072] (2) Add glycerin and sodium chloride to 95% of the total amount of water for injection to dissolve;
[0073] (3) After adding sodium bisulfite to the above solution to dissolve, add fructose to dissolve;
[0074] (4) Add 1mol / L sulfurous acid to the above solution to adjust the pH value to 4.2, add water for injection to the full amount, add activated carbon, stir and mix well, and measure the marked content of glycerin, sodium chloride, and fructose at 95.0-105.0%;
[0075] (5) Coarsely filter and finely filter the above medicinal solution, fill, seal, sterilize with damp heat at 121°C for 8 minutes, inspect...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com