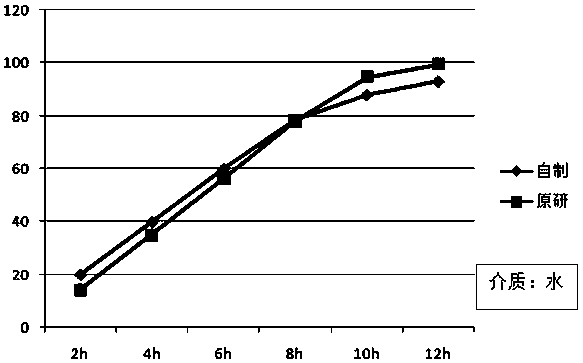
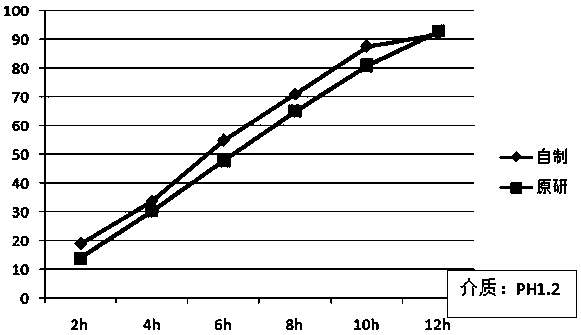
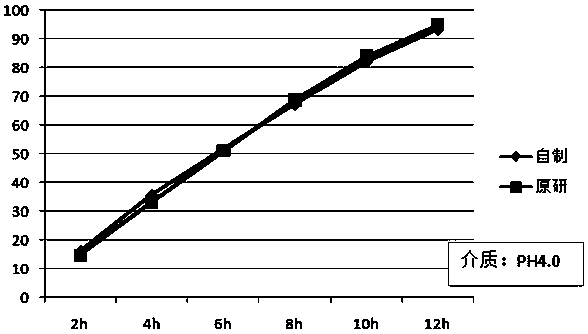
A kind of felodipine sustained-release tablet and preparation method thereof
A kind of sustained-release element tablets, gentle technology, applied in the direction of pharmaceutical formulations, cardiovascular system diseases, drug combinations, etc., can solve the problems of unfavorable preparation quality control, difficulty in rapid onset of effect, difficulty in achieving consistent dissolution, etc., to ensure the solubilization effect , improve quality monitoring, and ensure the effect of uniform dispersion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] 1. Prescription of Sustained Release Tablets: 1000 tablets
[0037] materials
Prescription amount (g)
Proportion (%)
effect
[0038] Felodipine
5
2.4
Main drug
RH40
10
4.8
Solubilizers
95% ethanol
Appropriate amount
Hypromellose E5
5
2.4
75% ethanol
Appropriate amount
120
58.0
filler
30
14.5
filler
silica
6
2.9
absorbent
Hypromellose K4M
30
14.5
Skeleton material
Sodium stearyl fumarate
1
0.5
[0039] 2. Preparation of Sustained-release Tablets:
[0040] (1) RH40 (i.e. polyoxyethylene 40 hydrogenated castor oil) is heated and dissolved in a water bath to liquid, and 95% ethanol of the prescribed amount is added, and felodipine is added in a stirring state to prepare a medicinal solution;
[0041] ...
Embodiment 2
[0053] 1. Prescription of Sustained Release Tablets: 1000 tablets
[0054] materials
Prescription amount (g)
Proportion (%)
effect
5
2.4
Main drug
RH40
5
2.4
Solubilizers
95% ethanol
Appropriate amount
solvent
Hypromellose E5
5.2
2.5
75% ethanol
Appropriate amount
solvent
90
43.5
filler
60
29.0
filler
kaolin
5
2.4
absorbent
Hypromellose K100
34.8
16.8
Skeleton material
2
1.0
[0055] 2. Preparation of Sustained-release Tablets:
[0056] The filler in Example 1 is replaced by mannitol and microcrystalline cellulose, the absorbent is replaced by kaolin, the skeleton material is replaced by hypromellose K100, the lubricant is replaced by sodium stearate, when granulating, drying and sizing The drying te...
Embodiment 3
[0062] 1. Prescription of Sustained Release Tablets: 1000 tablets
[0063] materials
Prescription amount (g)
Proportion (%)
effect
Felodipine
5
2.4
Main drug
RH40
5
2.4
Solubilizers
95% ethanol
Appropriate amount
solvent
Hypromellose E5
8.1
3.9
75% ethanol
Appropriate amount
solvent
101.4
49.0
filler
30
14.5
filler
silica
3.9
1.9
absorbent
Hypromellose E50
51.5
24.9
Skeleton material
2.1
1.0
lubricant
[0064] 2. Preparation of Sustained-release Tablets:
[0065] Replace the filler in Example 1 with mannitol and pregelatinized starch, replace the skeleton material with hypromellose E50, replace the lubricant with sodium stearate, and control the drying temperature at 40°C during granulation, drying and sizing , and all the ot...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com