Preparation method for copper and aluminium hydrotalcite
A hydrotalcite, copper-aluminum technology, applied in chemical instruments and methods, copper compounds, inorganic chemistry, etc., can solve the problems of high cost, large metal salt, low atom utilization rate, etc., and achieve low industrialization costs, good crystallinity, Effect of High Atom Utilization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Weigh 6g of AlCl 3 and 4.9g of Cu(OH) 2 Dissolve in 50ml of deionized water in a three-necked flask to form a mixed solution. Constant electric stirring at 30 °C. The solution turned blue in about 30 minutes. Measure its pH=3, continue to stir and react for 3h, then crystallize at 30°C for 24h, then filter it with suction, wash it with deionized water for 3 times, put it in a drying oven at 40°C for 24h, and grind it to obtain Cu / Al hydrotalcite powder.
Embodiment 2
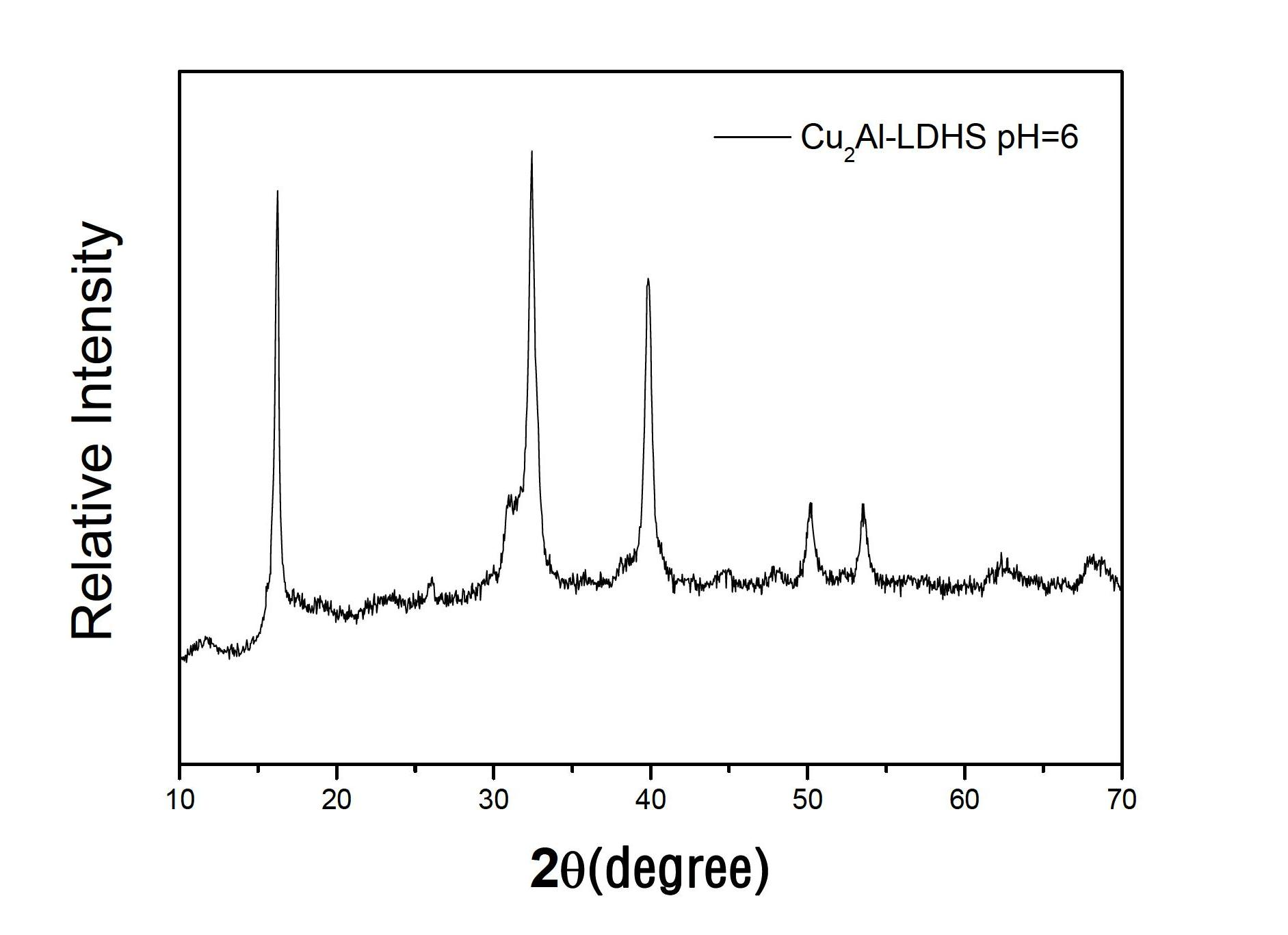
[0028] Weigh 6g of AlCl 3 and 4.9g of Cu(OH) 2 Dissolve in 50ml of deionized water in a three-necked flask to form a mixed solution. Under constant temperature electric stirring at 35°C, the solution turns blue in about 30 minutes. Weigh 5g of NaOH, dissolve it in 50ml of deionized water to make a 0.1g / ml solution, add part of it to the above blue solution, adjust pH=6, continue to stir for 3h and then crystallize at 35°C for 18h , and then suction filtered, and washed with deionized water for 3 times, then placed in a 40°C drying oven for 24 hours, and then ground to obtain Cu / Al hydrotalcite powder.
Embodiment 3
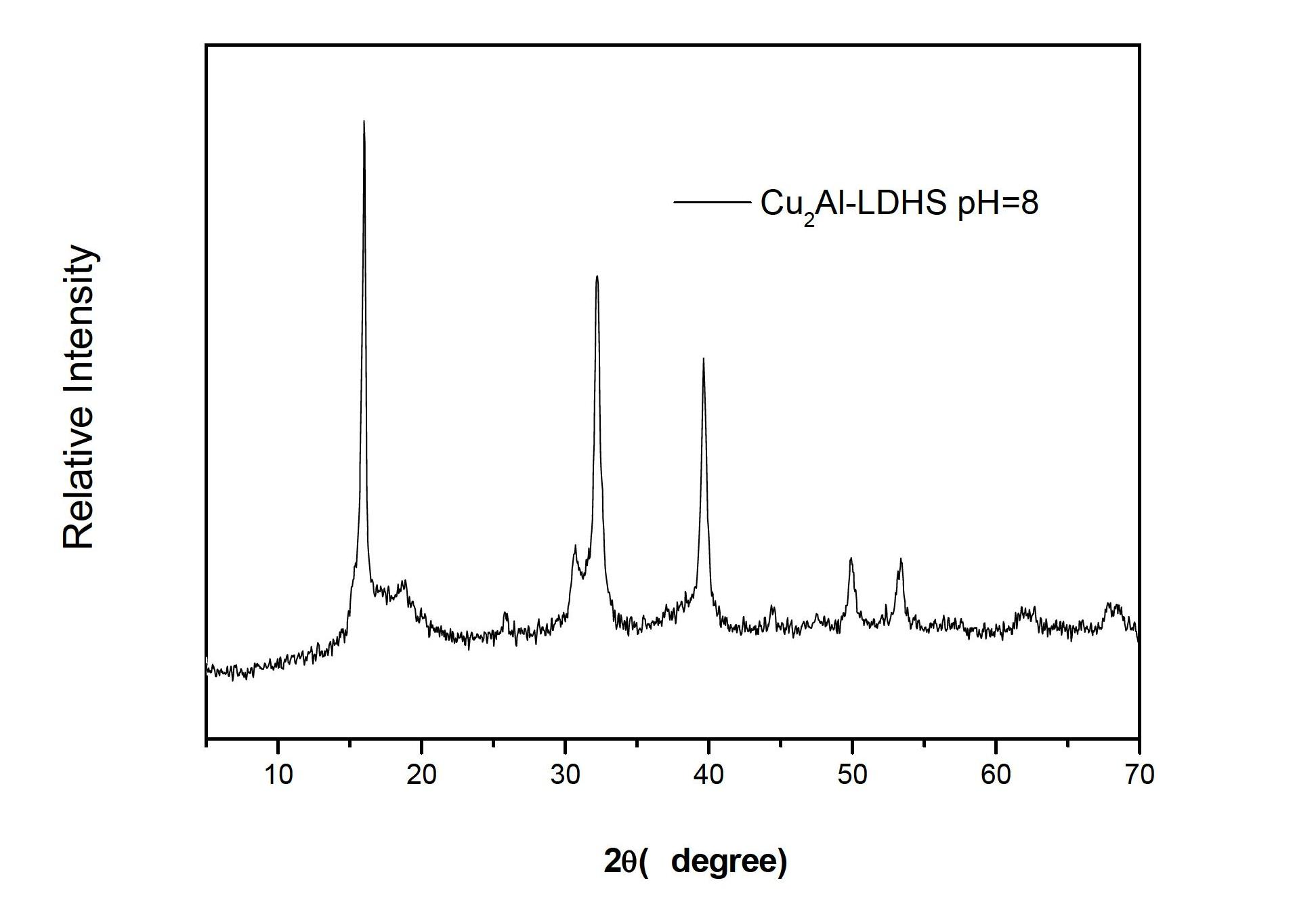
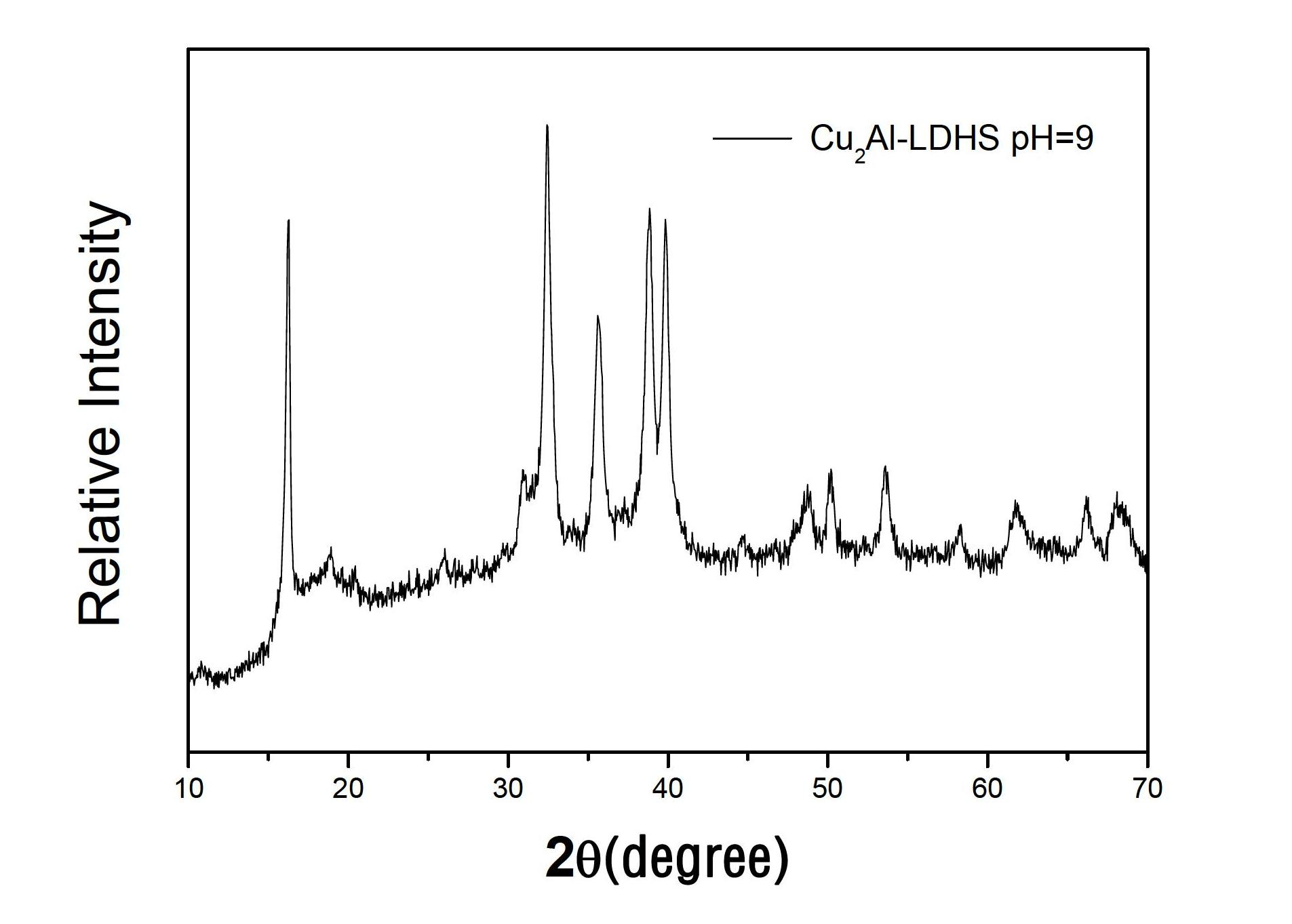
[0030] Weigh 6g of AlCl 3 and 4.9g of Cu(OH) 2 Dissolve in 50ml of deionized water in a three-necked flask to form a mixed solution. Under constant temperature electric stirring at 35°C, the solution turns blue in about 30 minutes. Weigh 5g of NaOH, dissolve it in 50ml of deionized water to make a 0.1g / ml solution, add part of it to the above blue solution, adjust pH=6, continue stirring for 3h and then crystallize at 40°C for 20h , and then suction filtered, and washed with deionized water for 3 times, then placed in a 60°C drying oven for 24 hours, and then ground to obtain Cu / Al hydrotalcite powder. Its XRD pattern is as follows figure 2 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com