Method for improving optimum temperature of family-10 xylanase
A technology of xylanase and family, applied in the field of bioengineering, can solve problems such as low thermal stability, difficult to meet application requirements, and low substrate specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] The construction of embodiment 1 mutant enzyme
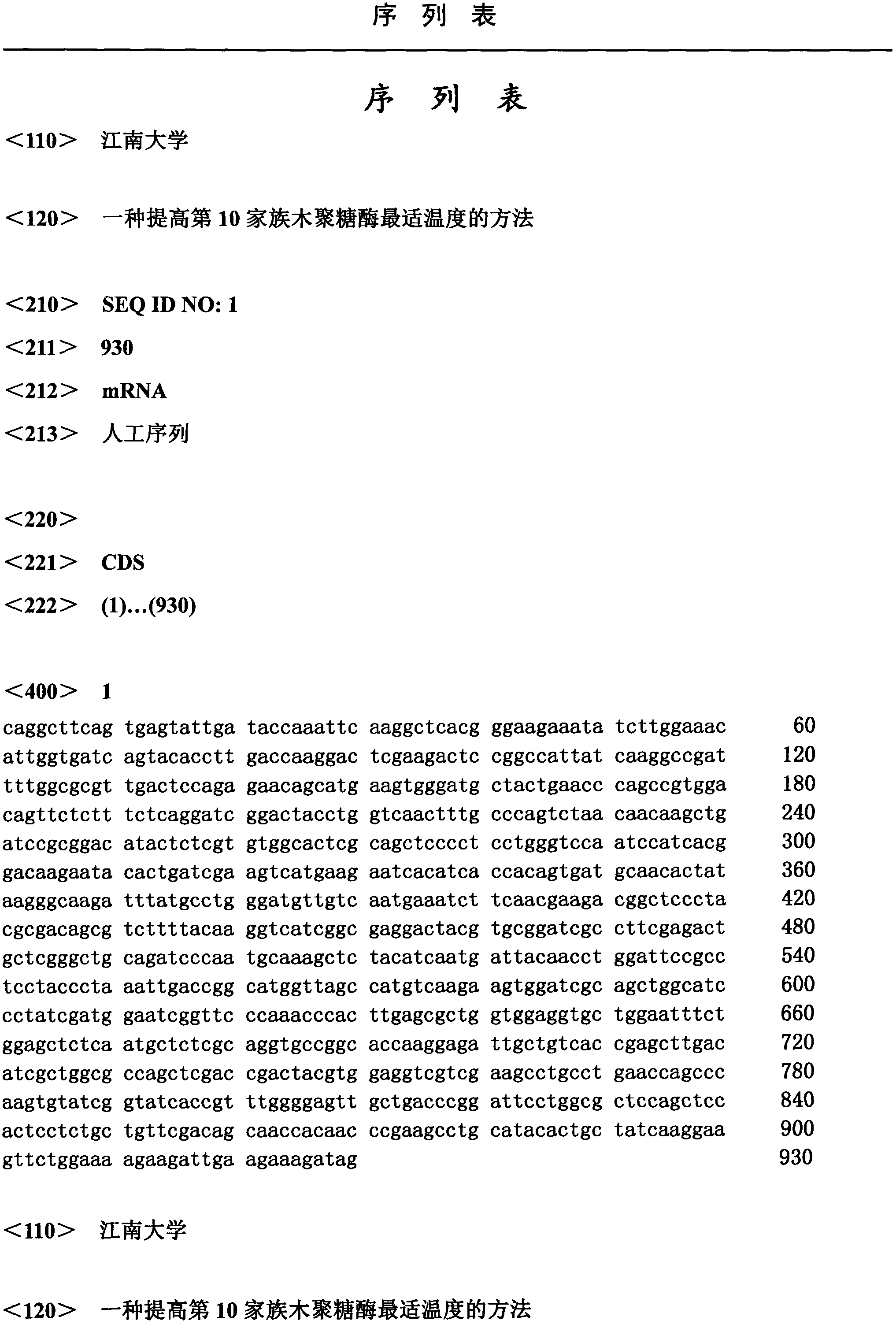
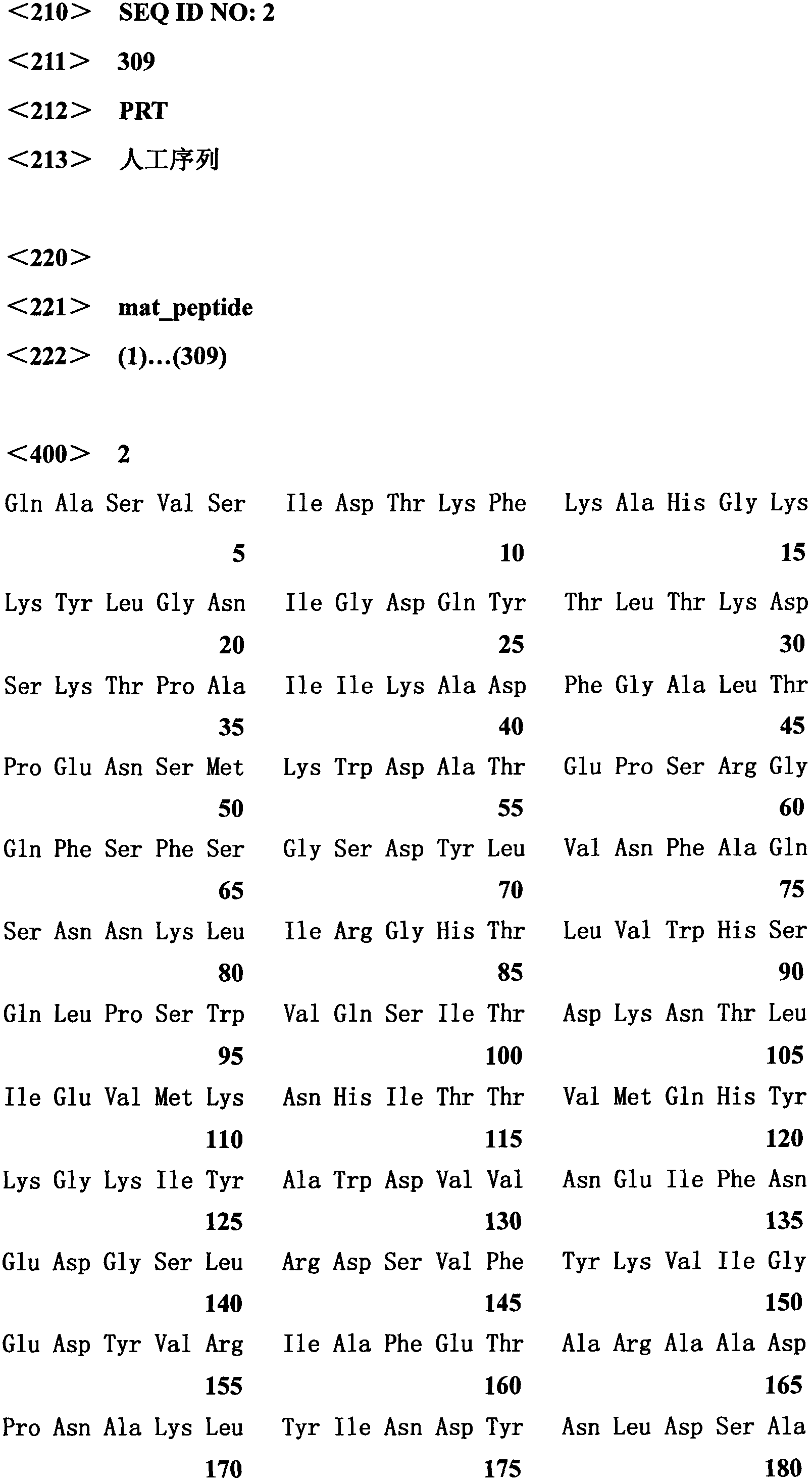
[0020] Extract the T vector (pUCm-T-Aus xyn10A) containing the Aus Xyn10A gene, and use the mature peptide primer Xyn10A-F1 (5'-GAATTCCAGGCTTCAGTGAGTATTGA-3') of the Aus Xyn10A gene as a template to carry out the first round of PCR, The reaction conditions are: 94°C for 5 minutes; 2 cycles of 94°C for 30s, 45°C for 30s, and 72°C for 70s; 28 cycles of 94°C for 30s, 55°C for 30s, and 72°C for 70s; 72°C for 10 minutes; Using the first-round PCR product as a template, the second-round PCR was carried out using primers Xyn10A-F1 and primer XynCHR2. The reaction conditions were: 94°C for 5 minutes; 2 cycles, 94°C for 30s, 45°C for 30s, and 72°C for 70s; 28 cycles Cycle 94°C for 30s, 55°C for 30s, 72°C for 70s; 72°C for 10min; store at 10°C. The two rounds of PCR amplification products were analyzed by 1% agarose gel electrophoresis, the target band was recovered by cutting the gel and ligated with the pUCm-T vector (pUCm-T-Aus...
Embodiment 2
[0021] Example 2 Containing the Construction of the Expression Plasmid Encoding Aus Xyn10A' Mature Peptide Gene
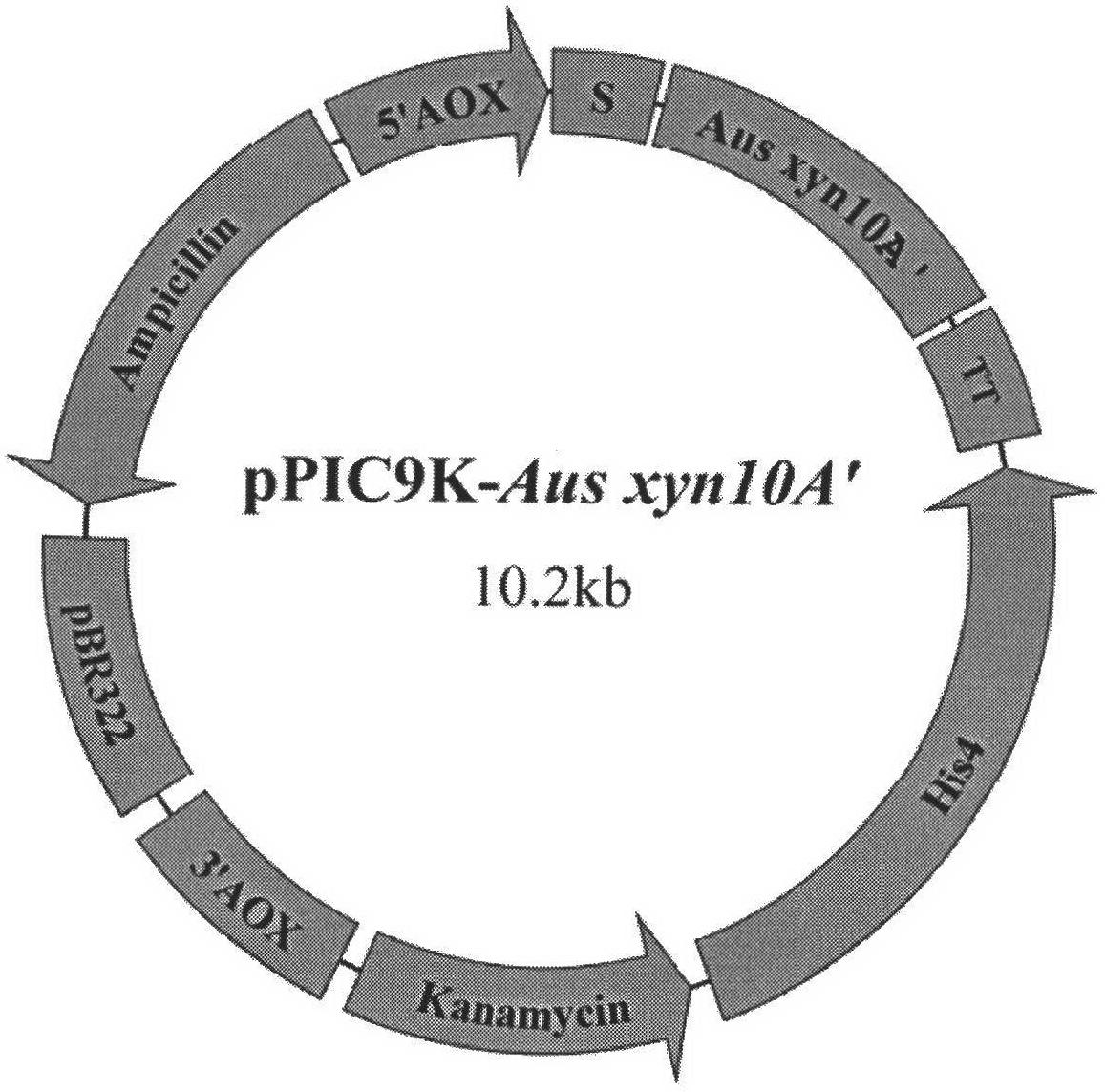
[0022] Use EcoR I and Not I to double-enzyme digest the target gene recovered from rubber tapping and pPIC9K respectively. The digestion time is 4 hours. The digested product recovered from rubber tapping is ligated overnight (>12 hours) under the action of T4 DNA ligase to obtain the recombinant plasmid pPIC9K -Ausxyn10A'( figure 1 ), and sequenced the recombinant expression plasmid.
Embodiment 3
[0023] Example 3 Construction, expression, product purification and activity determination of GS115 / Aus xyn10A'
[0024] Linearize pPIC9K-Aus xyn10A' with Sal I, perform electrotransformation and screening according to the Pichia expression manual, and obtain high-copy Pichia recombinant GS115 / Aus xyn10A'. The engineered bacterium was induced with 0.5% methanol for 72 hours, and the recombinant xylanase activity in the fermentation broth was measured by DNS method up to 24IU / mL. The centrifuged supernatant is the recombinant xylanase crude enzyme solution, which is concentrated by an ultrafiltration membrane with a molecular weight cut-off of 10kDa, and then purified by DEAE-Sepharose Fast Flow ion exchange chromatography and Sephadex G-75 gel filtration chromatography. After purification, It was detected as a single band by SDS-PAGE, and showed that the molecular weight of the recombinant xylanase was 42kDa. The optimum action temperature of the recombinant xylanase is 50°C,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com