Preparation method of non noble metal catalyst for cathodic oxygen reduction reaction of fuel cell
A fuel cell cathode, non-precious metal technology, applied in the field of non-precious metal catalyst preparation, can solve the problems of slow reaction speed, restricting the commercialization process of fuel cells, high price, etc., and achieves low cost, strong anti-poisoning ability and stability. Good results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
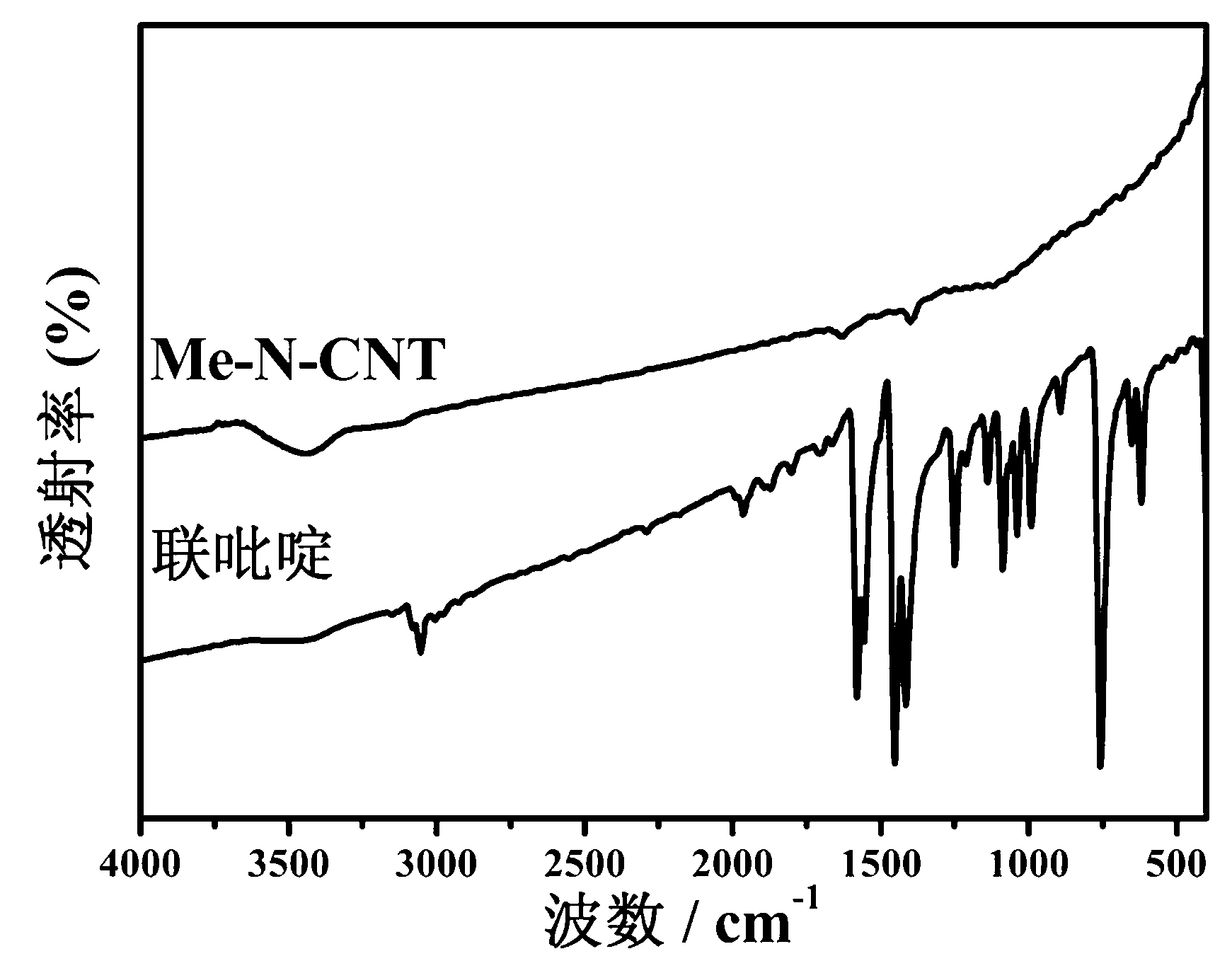
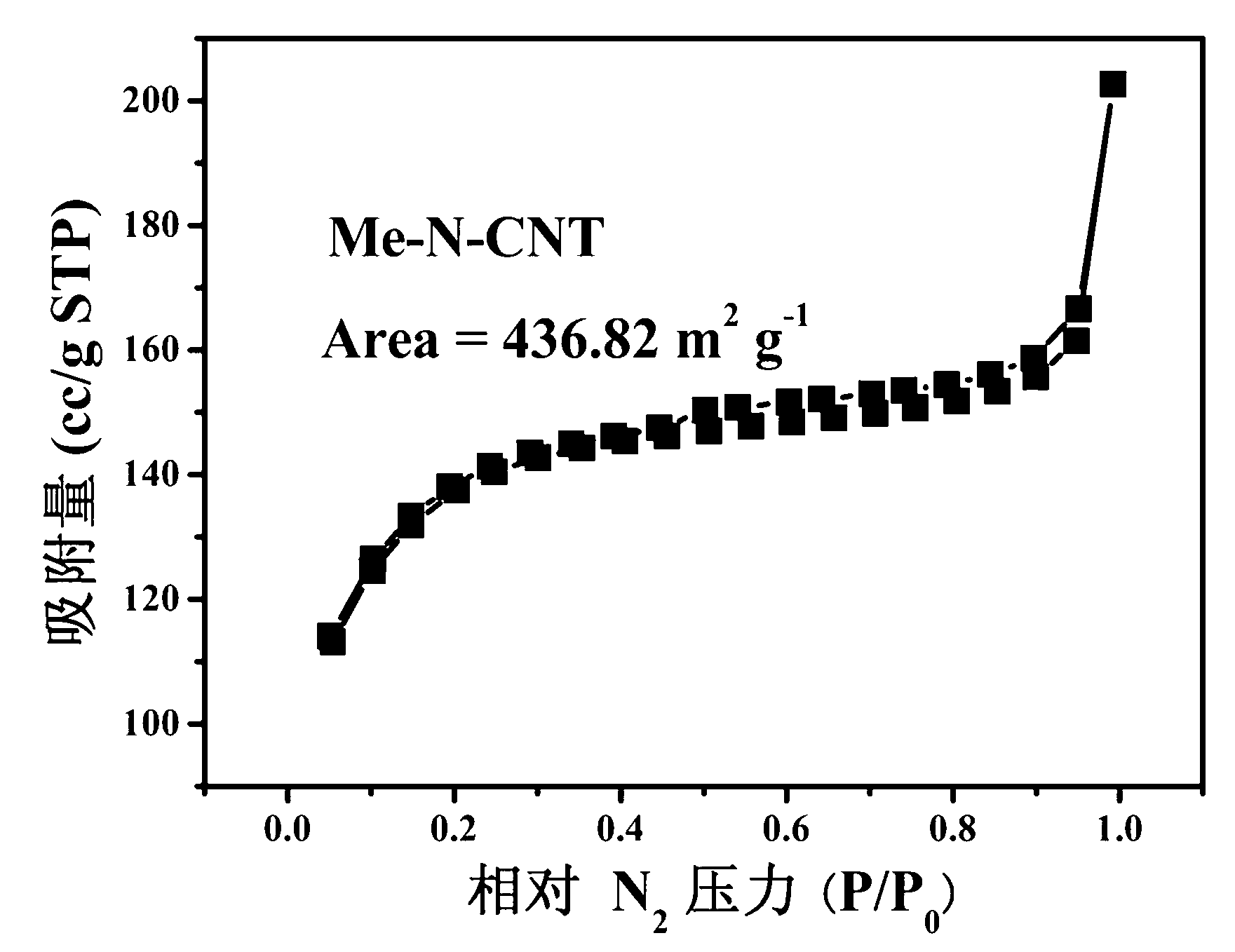
[0028] Add 40 ml methanol to a 100 mL flask, then add 3.2 g bipyridine, 0.5 g carbon nanotubes, ultrasonically disperse and stir for half an hour; add 0.5 g nickel nitrate solid and stir vigorously for about 2 hours, then steam on a rotary evaporator After drying, dry it in an oven and fully grind; then place it in a tube furnace with nitrogen, heat treatment at 600 ℃ for 2 hours, take it out, grind it, add it to a 0.5 M sulfuric acid solution, and treat it at 60 ℃ For 12 hours, filter with suction, wash with water, and dry to obtain a non-precious metal catalyst.
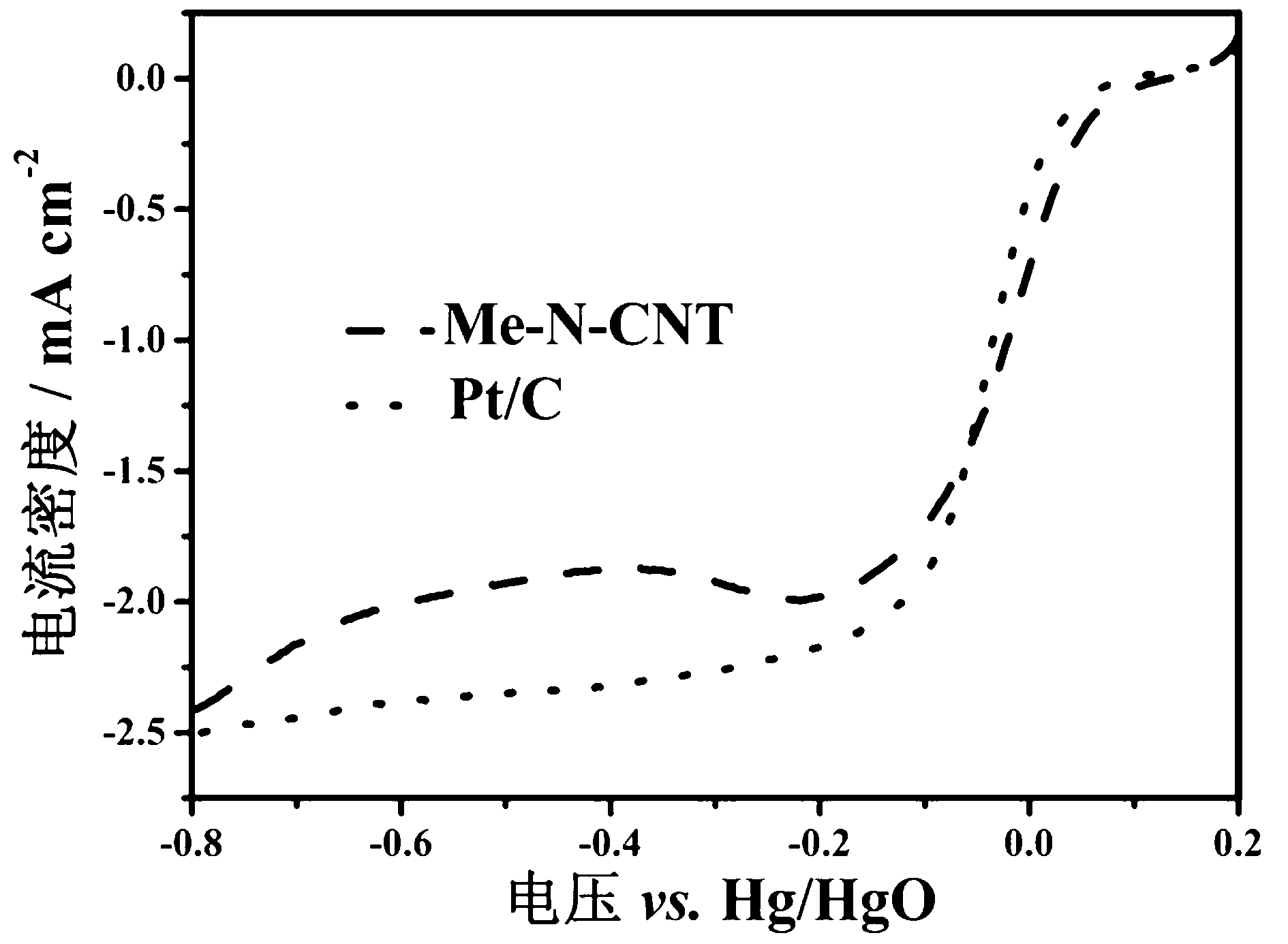
[0029] Compared with commercial platinum-carbon catalysts, non-precious metal catalysts have a 30 mV increase in half-potential. Under -0.2 V, the ratio of initial current to current after 20,000 s is 19% higher than that of commercial platinum-carbon catalysts.
Embodiment 2
[0031] Add 60 ml of methanol to a 100 mL flask, then add 2.2 g of bipyridine and 0.4 g of carbon nanotubes, ultrasonically disperse and stir for half an hour; add 0.5 g of nickel chloride solid, stir vigorously for 3 hours, and place on a rotary evaporator After evaporating the solvent, dry it in an oven and grind it thoroughly; then place it in a tube furnace, blow in nitrogen, heat-treat it at 650 ℃ for 1 hour, take it out, and grind; add it to a 0.5 M hydrochloric acid solution at 60 ℃ After treatment for 8 hours, suction filtration, water washing, and drying, a non-precious metal catalyst is obtained.
[0032] Compared with commercial platinum-carbon catalysts, non-noble metal catalysts have a 23 mV increase in half-potential. Under the condition of -0.2 V, the ratio of initial current to current after 20000 s is 17% higher than that of commercial platinum-carbon catalysts.
Embodiment 3
[0034] Add 60 ml of methanol to a 100 mL flask, then add 2.7 g of bipyridine, 0.3 g of carbon nanotubes, ultrasonically disperse and stir for half an hour, add 0.5 g of cobalt nitrate solid, stir vigorously for about 3.5 hours, and then evaporate After the instrument is evaporated to dryness, it is dried in an oven and fully ground; then it is placed in a tube furnace with nitrogen gas, heat treated at 750 ℃ for 4 hours, taken out, ground, and added to a 2 M nitric acid solution. Treated at 80°C for 16 hours, filtered with suction, washed with water, and dried to obtain a non-precious metal catalyst.
[0035] Compared with commercial platinum-carbon catalysts, non-noble metal catalysts have an increase of 38 mV in half-potential. Under the condition of -0.2 V, the ratio of initial current to current after 20000 s is 12% higher than that of commercial platinum-carbon catalysts.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com