Method for preparing metal-modified alpha type molybdenum carbide catalyst and application of metal-modified alpha type molybdenum carbide catalyst in low-temperature water-gas shift reaction
A technology of metal modification and molybdenum carbide, applied in chemical instruments and methods, non-metallic elements, inorganic chemistry, etc., can solve the problems of α-MoC that have not yet been seen, and achieve the effect of simplified preparation process, simple process and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
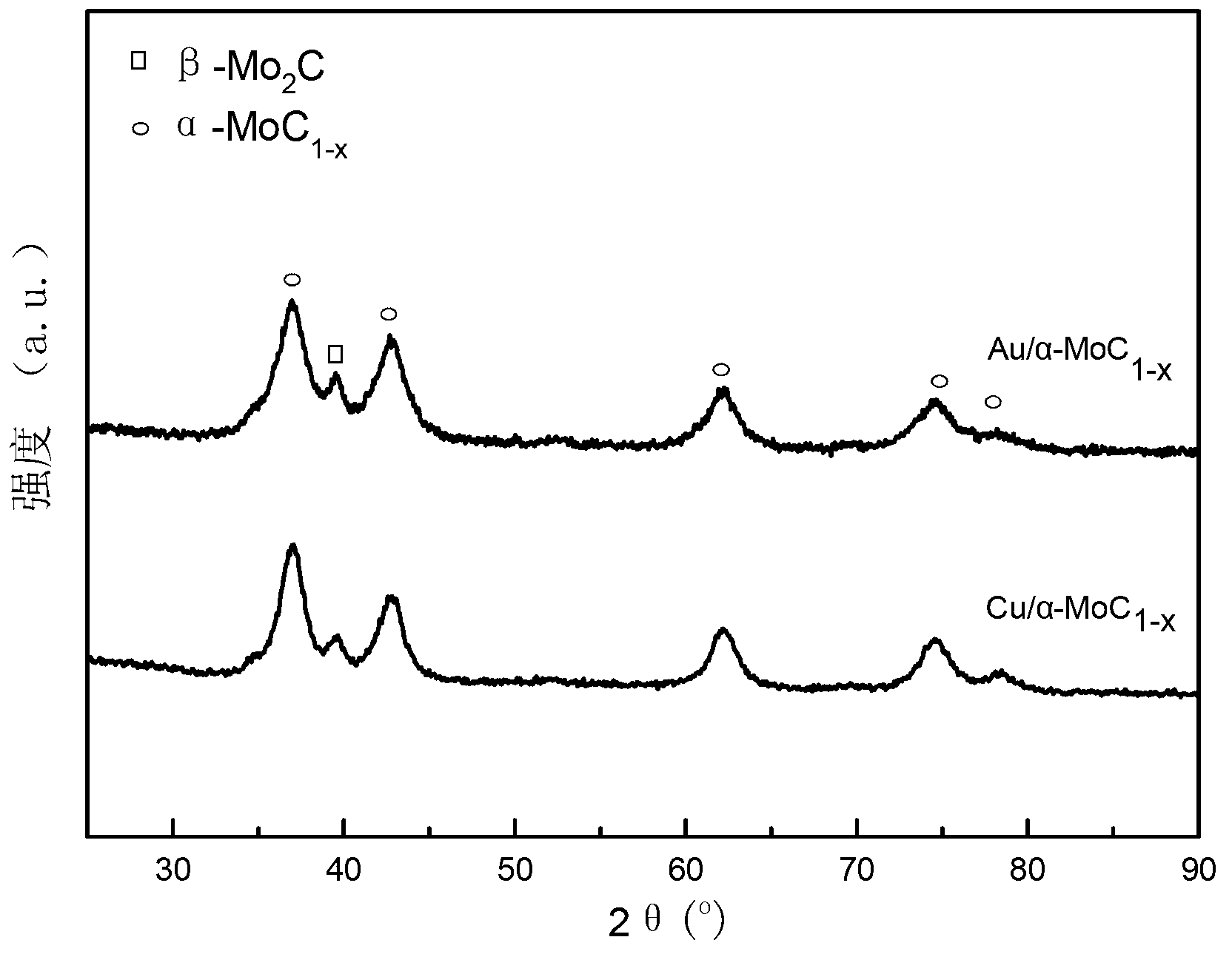
[0023] Preparation of Au-modified α-molybdenum carbide catalyst samples: (1) 3.531 g (NH 4 ) 6 Mo 7 o 24 4H 2 O was dissolved in 15 ml deionized water to make a solution, and 13.18 ml HAuCl 4 (0.02428 mmol / mL) was added to the ammonium molybdate solution, and the above mixed solution was stirred at room temperature for 4 h, then changed to 80 °C water bath and stirred until the solution evaporated completely to obtain a solid precipitate, which was dried at 110 °C for 12 h, and calcined at 500 °C for 4 h to obtain the oxide precursor. (2) Press the precursor prepared above into 40-60 meshes, weigh the samples required for the reaction and place them in a quartz reactor, in 20% CH 4 / H 2 Carry out temperature-programmed carbonization in a mixed atmosphere, from room temperature to 300 °C, the heating rate is 5 °C / min, and then from 300 °C to the final carbonization temperature of 700 °C, the heating rate is 1 °C / min, at the final carbonization temperature, Constant tempe...
Embodiment 2
[0025] Preparation of Cu-modified α-molybdenum carbide catalyst samples: (1) Weigh 0.2395 g Cu(NO 3 ) 2 ·3H 2 O and 3.531 g (NH 4 ) 6 Mo 7 o 24 4H 2 O was dissolved in 5 ml and 15 ml deionized water respectively to make a solution, and the two solutions were mixed. Stir the above mixed solution at room temperature for 4 h, change to a water bath at 80 °C and stir until the solution evaporates completely to obtain a solid precipitate, which is dried at 110 °C for 12 h and calcined at 500 °C for 4 h to obtain the oxide precursor . (2) The carbonization process is the same as in Example 2, and Cu / α-MoC is finally obtained 1-x catalyst.
Embodiment 3
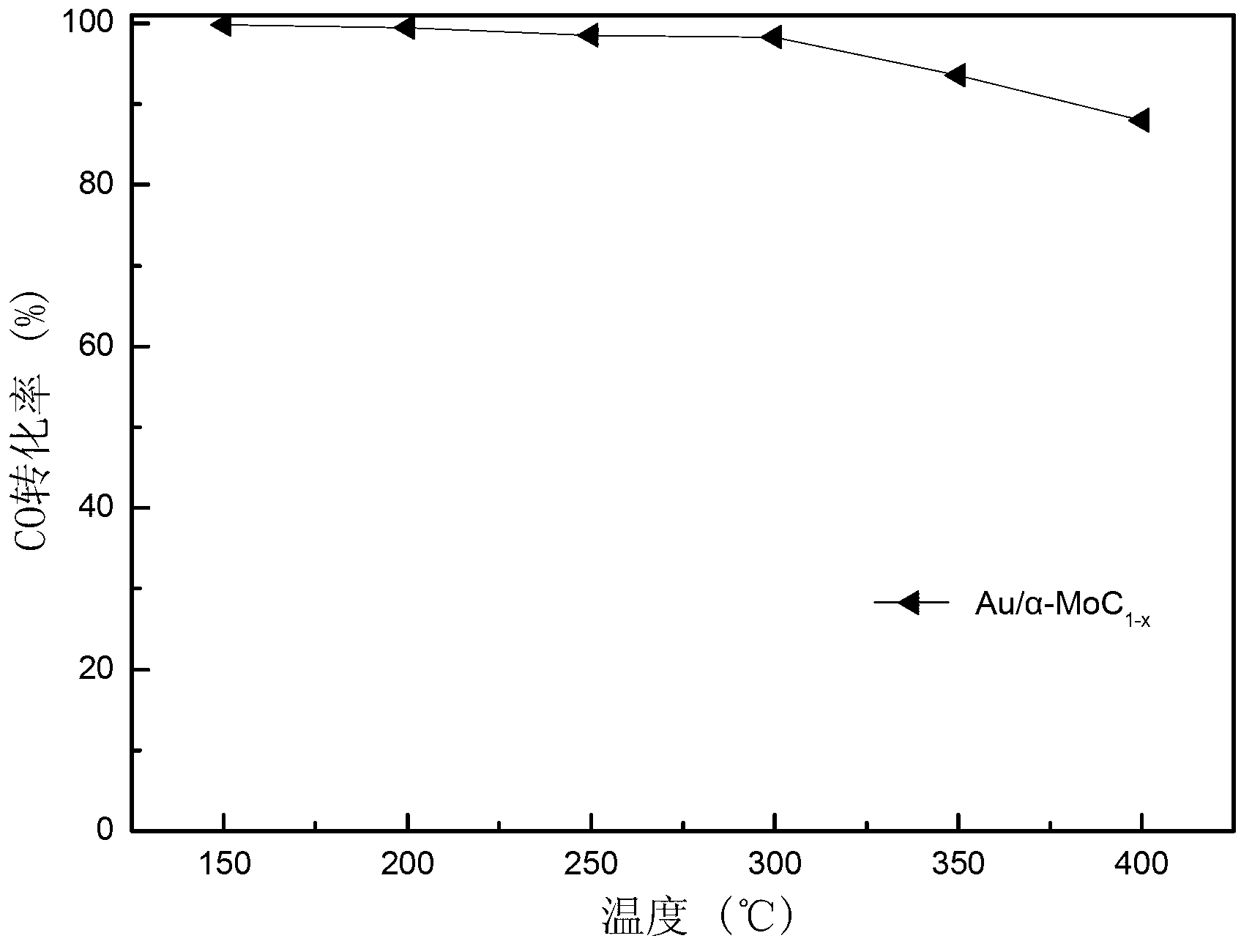
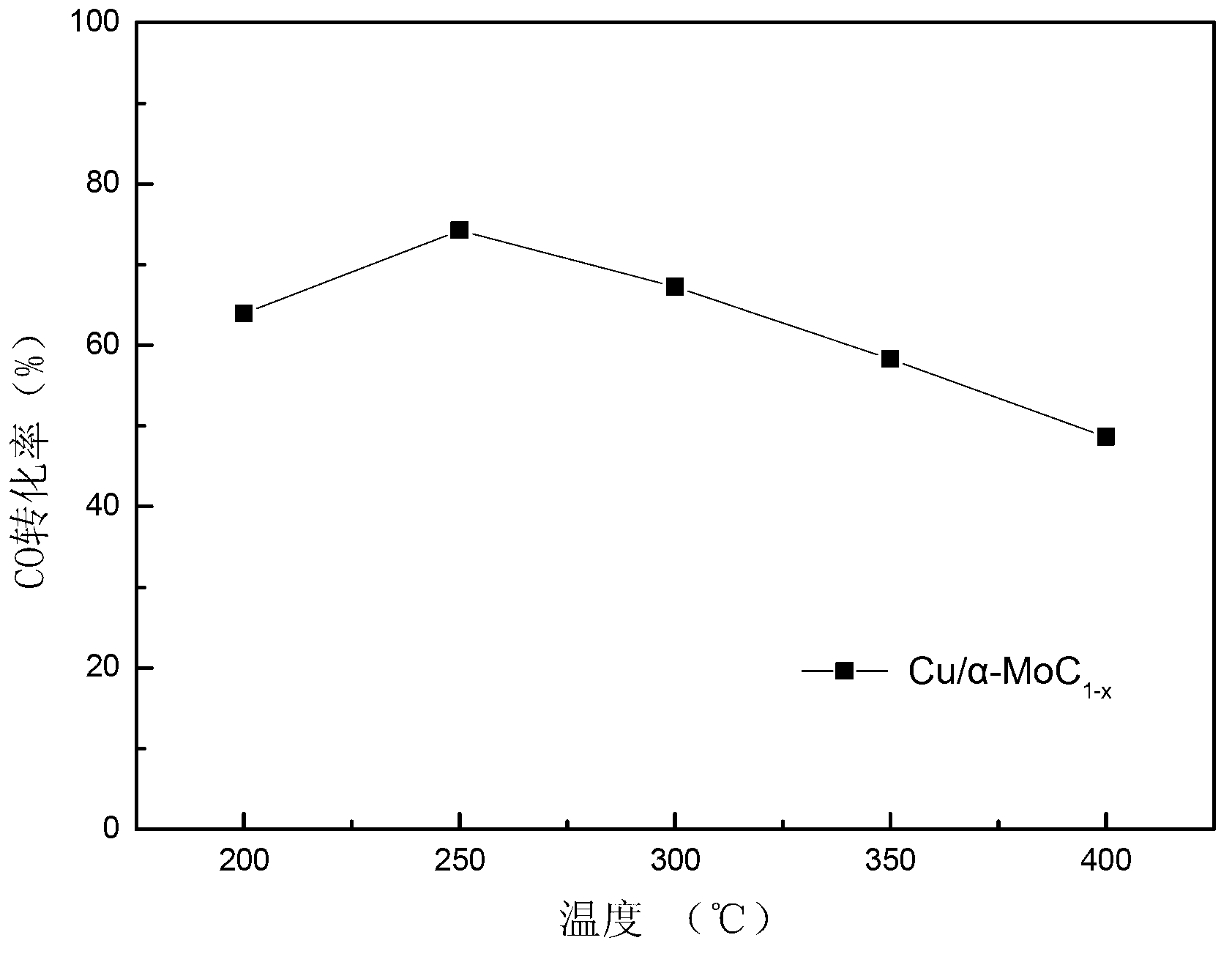
[0027] The evaluation of the activity of the above two metal-modified α-type molybdenum carbide catalysts was carried out on a self-made miniature fixed-bed catalytic reaction device. A quartz tube with an inner diameter of 6 mm was used as the reactor, and the activity evaluation conditions were: normal pressure, the loading amount of the catalyst composite oxide precursor (40-60 mesh) was 0.4 g, and the original test was carried out as described in step 2 in Example 1. In-situ carbonization, after the carbonization is completed, switch to the reaction gas for in-situ reaction under the protection of argon. The reaction gas composition is: 10.5% CO, 21% H 2 O, the rest is Ar (Ar is the balance gas), the total flow rate is 120 ml / min, and the mass space velocity is 18000 ml g -1 h -1 , The reaction temperature is 150 ℃ ~ 400 ℃. The content of CO in the tail gas was analyzed online by a Timex GC7890Ⅱ gas chromatograph equipped with a thermal conductivity detector.
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com