Method for preparing rutile titanium dioxide
A titanium dioxide, rutile type technology, applied in the direction of titanium dioxide, titanium oxide/hydroxide, etc., can solve the problems of increasing production cost and environmental pollution, and achieve the effect of protecting the environment, reducing production cost and reducing energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] A preparation method for rutile titanium dioxide, comprising the steps of:
[0033] Under the condition of stirring at 100r / min, add 0.62mL of titanium tetrachloride dropwise to 56mL of distilled water, transfer the resulting mixture to a polytetrafluoro container with a volume of 70mL, and then place the polytetrafluoro container in a stainless steel reaction kettle , Seal the kettle; the reaction kettle was kept at 50°C for static heat preservation for 24 hours. The obtained product was subjected to solid-liquid separation, and the liquid was recovered for use; the solid was washed and dried to obtain rutile-type titanium dioxide (sample S-1). After testing, the mass was 0.41 g, and the yield was 90%.
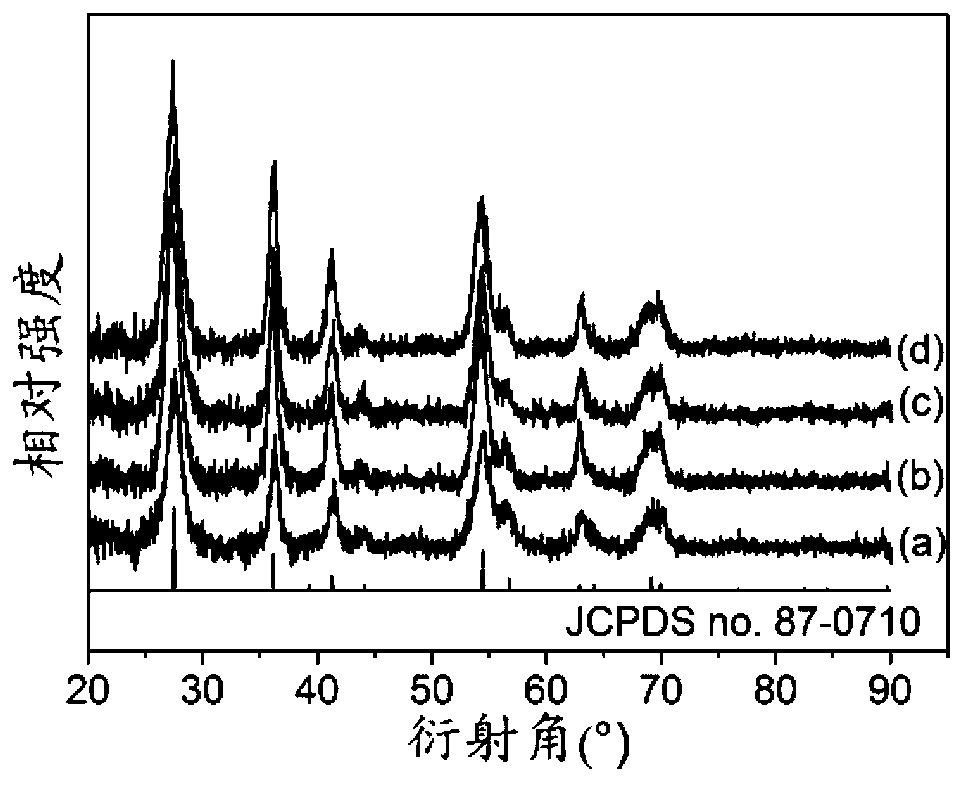
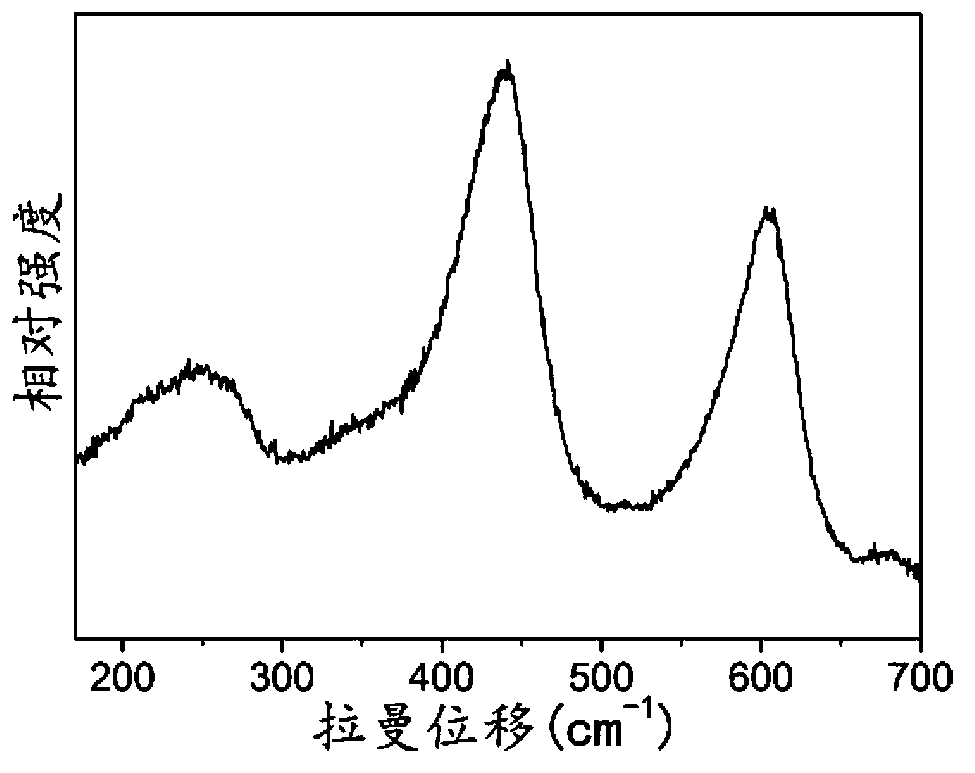
[0034] The XRD ( figure 1 , curve (a)) analysis results show that sample S-1 is pure rutile titanium dioxide. The Raman spectrum of the obtained sample S-1 ( figure 2 ) The results further show that the sample is pure rutile titanium dioxide.
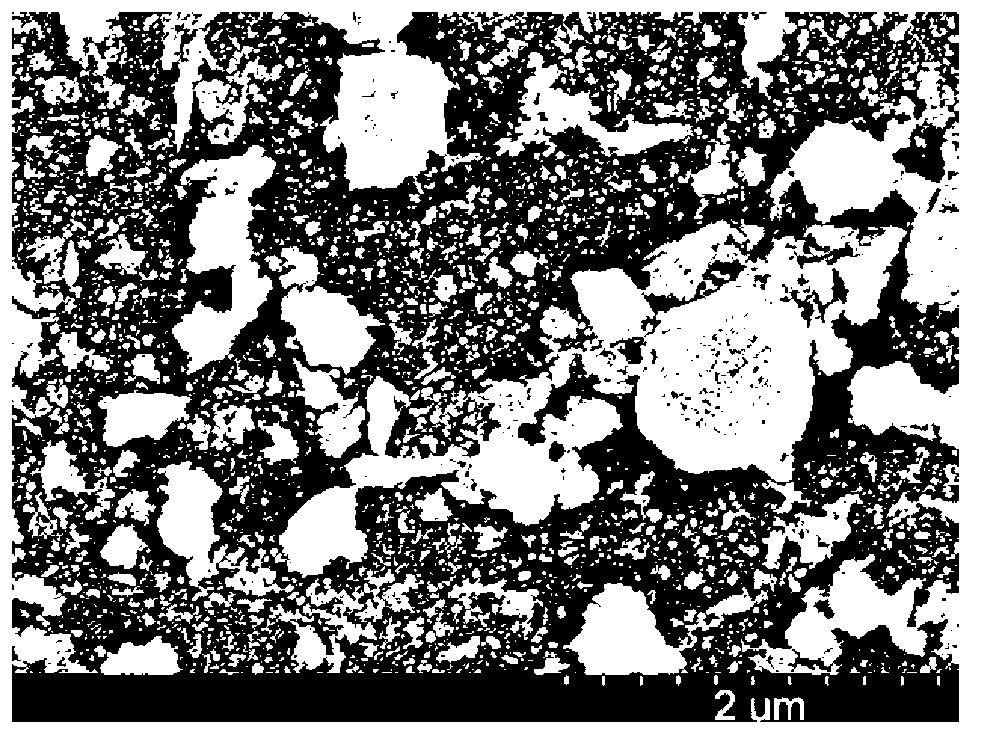
[0035] The scanning e...
Embodiment 2
[0037] A preparation method for rutile titanium dioxide, comprising the steps of:
[0038] Under the condition of stirring at 200r / min, 4.94mL of titanium tetrachloride was added dropwise to the liquid recovered in Example 1, and the resulting mixture was transferred to a polytetrafluoro container with a volume of 70mL, and the polytetrafluoro container was placed in In the stainless steel reaction kettle, seal the kettle; the reaction kettle was kept at 75°C for static heat preservation for 6 hours. The obtained product was subjected to solid-liquid separation, and the liquid was recovered for use; the solid was washed and dried to obtain rutile-type titanium dioxide (sample S-2). After testing, the mass was 2.74 g, and the yield was 76%.
[0039] The XRD ( figure 1 , curve (b)) analysis results show that sample S-2 is pure rutile titanium dioxide.
[0040] The scanning electron micrograph of gained sample S-2 sees Figure 4 .
Embodiment 3
[0042] A preparation method for rutile titanium dioxide, comprising the steps of:
[0043] Under the condition of stirring at 300r / min, 4.94mL of titanium tetrachloride was added dropwise to 56mL of ethanol aqueous solution (absolute ethanol / distilled water = 3:7, volume ratio), and the resulting mixture was transferred to a 70mL polytetrafluoroethylene In the container, the polytetrafluoroethylene container was placed in a stainless steel reaction kettle, and the kettle was sealed; the reaction kettle was kept at 65°C for static heat preservation for 24 hours. The obtained product was subjected to solid-liquid separation, and the liquid was recovered for use; the solid was washed and dried to obtain rutile-type titanium dioxide (sample S-3). After testing, the mass was 3.46 g, and the yield was 96%.
[0044] The XRD ( figure 1 , curve (c)) analysis results show that sample S-3 is pure rutile titanium dioxide.
[0045] The scanning electron micrograph of gained sample S-3 se...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com