Method for preparing sulfur ether intermediates of proton pump inhibitor
A technology of agent sulfide and intermediate, applied in the field of pharmaceutical manufacturing, can solve problems such as unfavorable cost and complicated operation, and achieve the effects of avoiding oxidative deterioration, concise reaction steps and reasonable process route design.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
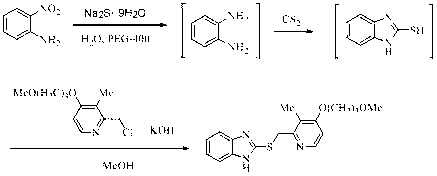
[0065] Proton pump inhibitor temoprazole sulfide intermediate 2-[ S -(pyridin-2-yl)methyl]thio-1 H - the preparation of benzimidazole
[0066]
[0067] 150 mL of water was added to the reaction flask, and 2-nitroaniline (13.8 g, 0.1 mol), Na 2 S·9H 2 O (72 g, 0.3 mol) and PEG-400 (2.0 mL), heated to reflux, reacted for 4 hours, then cooled the reaction system to 40 °C, directly added CS without separation 2 (9.0 mL, 0.15 mol), after continuing to reflux for 7 hours, add 70 mL methanol, 2-chloromethylpyridine (15.3 g, 0.12 mol), KOH (13.2 g, 85%, 0.2 mol) , heated to reflux again, and reacted for 4 hours. The reaction solution was evaporated under reduced pressure to remove methanol, poured into 100 mL of ice water, a large amount of solids were precipitated, and the yellow crude product was obtained by suction filtration, which was recrystallized with petroleum ether to obtain 15.3 g of light yellow solid, yield 64.6%, mp 105~108 ℃.
[0068] Spectral data: EI-MS ( m...
Embodiment 2
[0071] Proton pump inhibitor Lansoprazole sulfide intermediate 2-[ S -(3-Methyl-4-(2,2,2-trifluoro)ethoxypyridin-2-yl)methyl]thio-1 H - the preparation of benzimidazole
[0072]
[0073] 150 mL of water was added to the reaction flask, and 2-nitroaniline (13.8 g, 0.1 mol), Na 2 S·9H 2 O (72 g, 0.3 mol) and PEG-400 (2.0 mL), heated to reflux, reacted for 4 hours, then cooled the reaction system to 40 °C, directly added CS without separation 2 (9.0 mL, 0.15 mol), after continuing to reflux for 7 hours, add 70 mL methanol, 2-chloromethyl-3-methyl-4-(2,2,2-trifluoro)ethoxy Basepyridine (28.8 g, 0.12 mol), KOH (13.2 g, 85%, 0.2 mol), heated to reflux again, and reacted for 4 hours. The reaction solution was evaporated under reduced pressure to remove methanol, poured into 100 mL of ice water, a large amount of solids precipitated, and the yellow crude product was obtained by suction filtration, which was recrystallized with petroleum ether to obtain 26.5 g of light yellow so...
Embodiment 3
[0078] 150 mL of water was added to the reaction flask, and 2-nitro-4-methoxyaniline (16.8 g, 0.1 mol), Na 2 S·9H 2 O (72 g, 0.3 mol) and PEG-400 (2.0 mL), heated to reflux, reacted for 5 hours, then cooled the reaction system to 40 °C, directly added CS 2 (9.0 mL, 0.15 mol), after continuing to reflux for 5.5 hours, 70 mL of methanol, 2-chloromethyl-3,5-dimethyl-4-methoxypyridine (22.3 g, 0.12 mol), KOH (13.2 g, 85%, 0.2 mol), heated to reflux again, and reacted for 4 hours. The reaction liquid was evaporated to remove methanol under reduced pressure, poured into 100 mL of ice water, a large amount of solids precipitated, and the yellow crude product was obtained by suction filtration, which was recrystallized with petroleum ether to obtain 23.7 g of light yellow solid, yield 71.9%, mp 118~119 ℃.
[0079] Spectral data: EI-MS ( m / z ): 329 [M + ];
[0080] 1 H-NMR (400 MHz, CDCl 3 , ppm), 8.21 (1H, s, pyridine), 7.42 (1H, d, J = 8.8 Hz, ArH), 7.10 (1H, d, J = 2....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com