Preparation method of HLA-A0201-restricted anti-HPV (human papillomavirus) antigen-specific CTL
A 1. HLA-A0201, restricted technology, applied in the field of biotechnology development and application research, can solve the problems of unknown, long time for in vitro separation and amplification, increase pollution, etc., and achieve the effect of easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
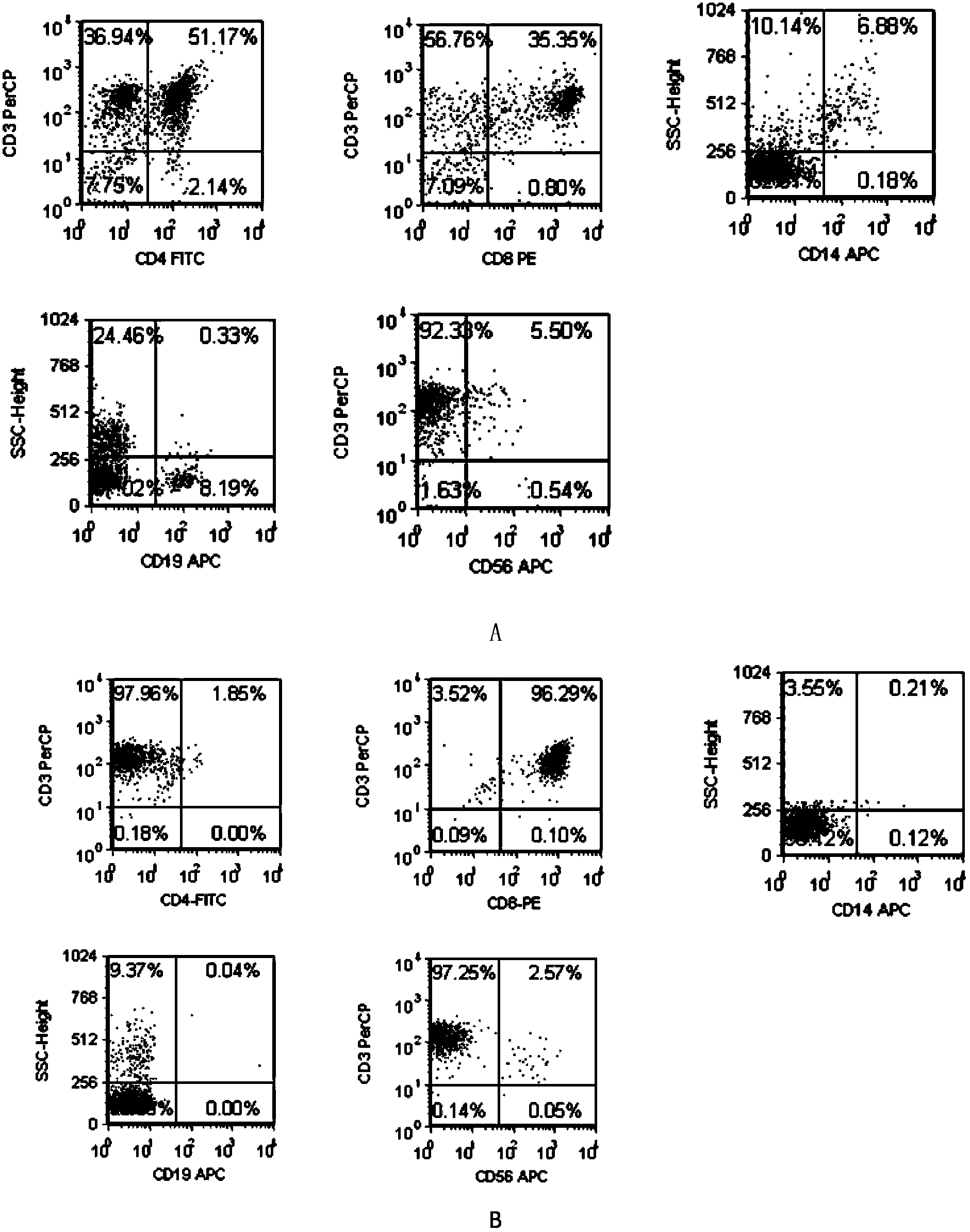
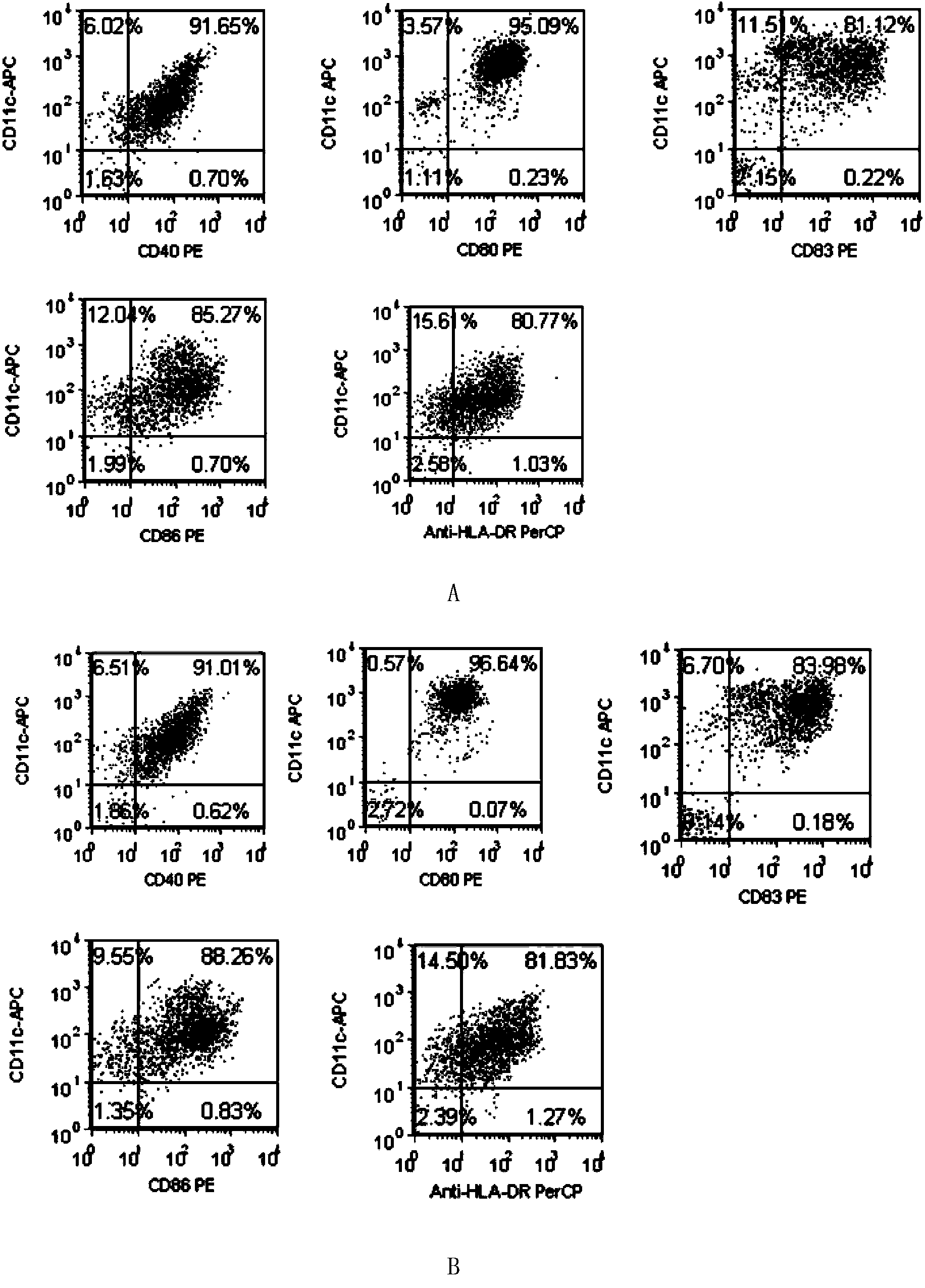
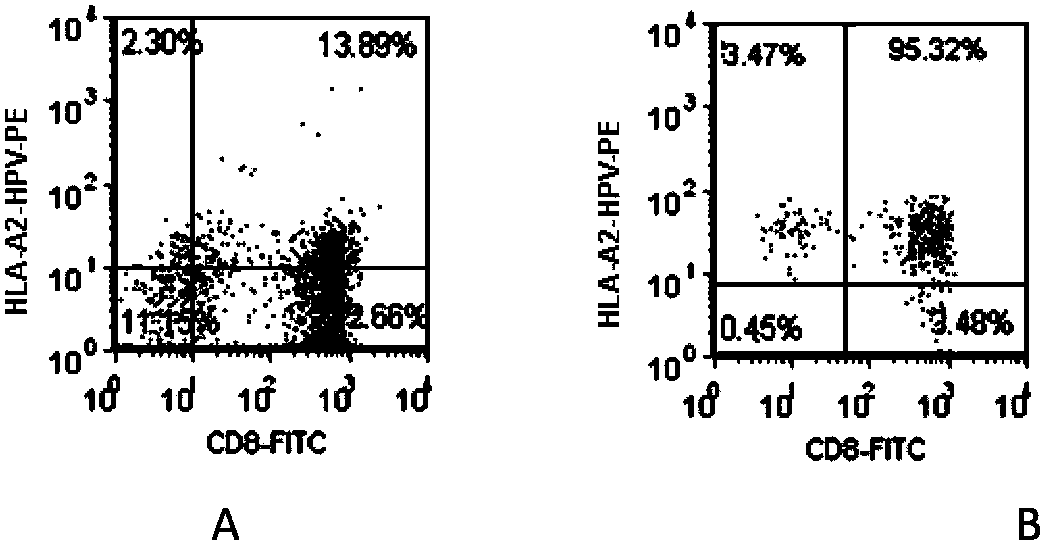
[0142] Example 1: HPV16E7 11-20 Antigen-specific CTL preparation
[0143] 1. Preparation for patients with HLA-A201+HPV16+cervical cancer
[0144] Take 1ml of peripheral anticoagulant blood from the patient, add FITC-labeled HLA-A2-mAb (American BD Company, product number 551285, the same below), and detect the expression of HLA-A2 (abbreviation of HLA-A201, the same below) by flow cytometry; Cancer patients were tested for HPV, and HPV16-positive patients were selected. Cervical cancer patients with simultaneous expression of HLA-A2 and HPV16 were selected.
[0145] 2. Antigen peptide synthesis
[0146] HPV16E7 polypeptide, the position is 11-20, and the sequence is YMLDLQPETV (SEQ ID NO.1) 10 peptide (hereinafter referred to as HPV16E7 11-20 Polypeptide), chemically synthesized (Shanghai Gill Biochemical Co., Ltd.), fully dissolved in sterile double distilled water, the peptide concentration is 5mg / ml, and stored in -80°C in aliquots.
[0147] 3. PBMC collection
[014...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com