Activated cathode for hydrogen evolution
一种阴极、阴极基底的技术,应用在电极、电极涂料、多种成分组成的覆层等方向,能够解决催化剂元素消耗、电解过程劣化、过电压性能变差等问题,达到保留率升高、电解性能优异、提高性质的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0090] A nickel mesh was used as the cathode substrate. The surface of the substrate was sufficiently roughened with alumina particles, and then immersed in an etching solution for 5 minutes. Prepare the etching solution as follows: mix 36% HCl with an equal amount of purified water, boil once and cool to 25 °C.
[0091] To prepare the coating solution, dinitrodiammine platinum(II) HNO 3 solution, dinitrodiammine palladium (II) HNO 3 solution and cerium(III) nitrate hexahydrate to obtain liquid concentrations of 20.8 g / l, 14.4 g / l and 34.0 g / l, respectively.
[0092] Using a roller, apply the coating solution on the nickel mesh so that the coating amount of Pt and Pd is 0.6 g / m per time 2 (Herein, the coating amount of noble metal is referred to as NM coating amount). The nickel mesh was dried at 60°C for 10 minutes and fired in an electric furnace at 500°C for 13 minutes. Repeat the above treatment 4 times so that the final NM coating amount of the catalyst layer is 2.6g...
Embodiment 2~8
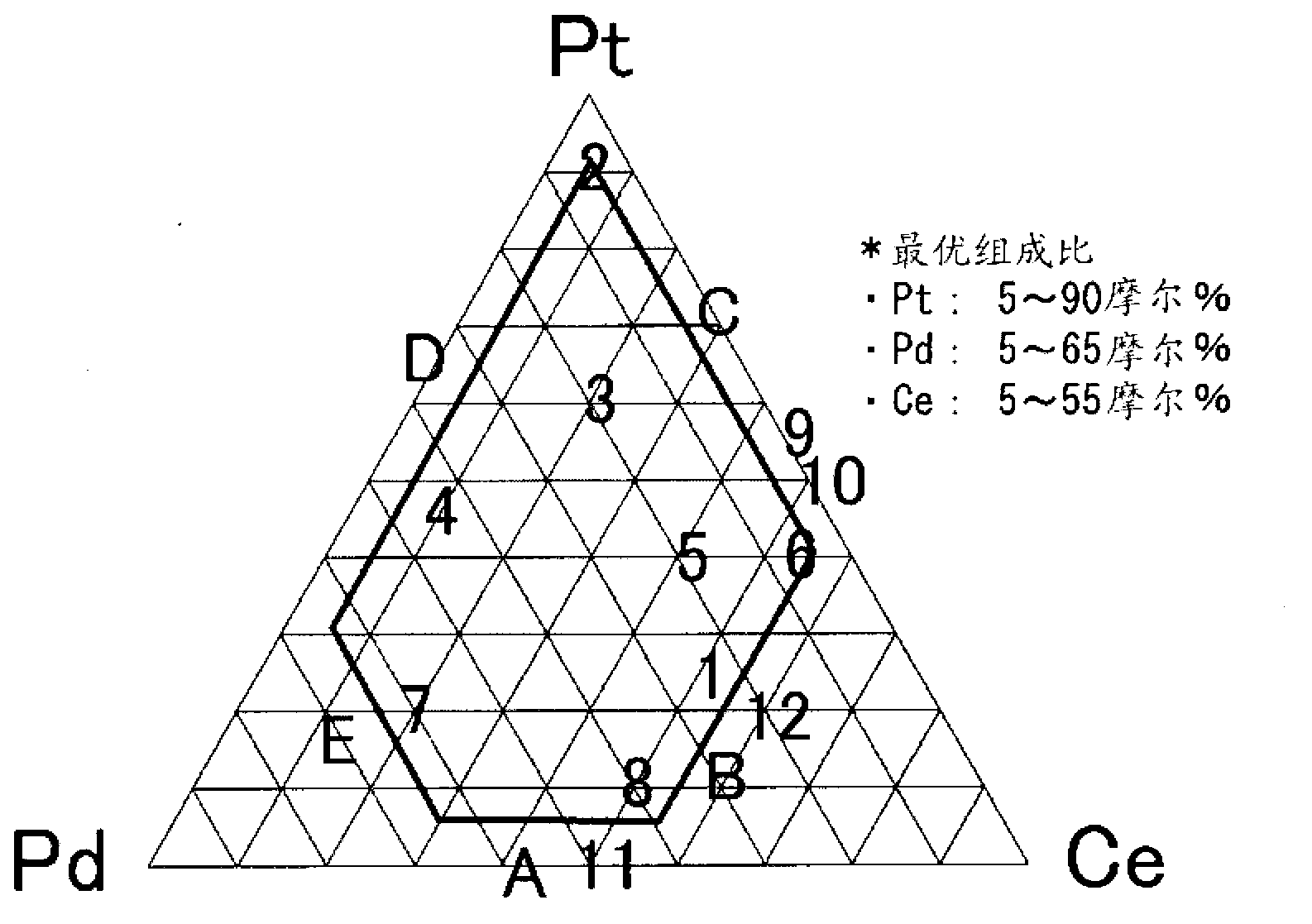
[0097] In the cathode samples No. 2 to 8 for hydrogen production, the composition ratio of the 3-element type catalyst including platinum (Pt), palladium (Pd) and cerium (Ce) and the amount of NM coating were changed, in addition, A cathode sample for hydrogen production was prepared in the same manner as in Example 1. In cathode samples Nos. 2 to 8 for hydrogen production, the composition ratio of platinum (Pt) was gradually decreased. The test results are shown in Table 1. In all samples, at 5kA / m 2 When the initial overvoltage is lower than 90mV.
[0098] These cathode samples No. 2-8 for hydrogen production all showed good results in the retention rate of the catalyst element after the short-circuit test and the high current density test, like the cathode sample No. 1 for hydrogen production. In particular, the retention of platinum (Pt), which is the most important element for keeping the hydrogen overvoltage low, is very high, and long-term hydrogen with low hydrogen ...
Embodiment 9 and 10
[0111] Cathode samples No. 13 and No. 14 for hydrogen production had different composition ratios of a 4-element type catalyst including platinum (Pt), palladium (Pd), cerium (Ce) and lanthanum (La). Except for this, these cathode samples for hydrogen production were prepared in the same manner as in Example 1.
[0112] The results are shown in Table 3. at 5kA / m 2 The initial overvoltages were 82mV and 83mV, both lower than 90mV.
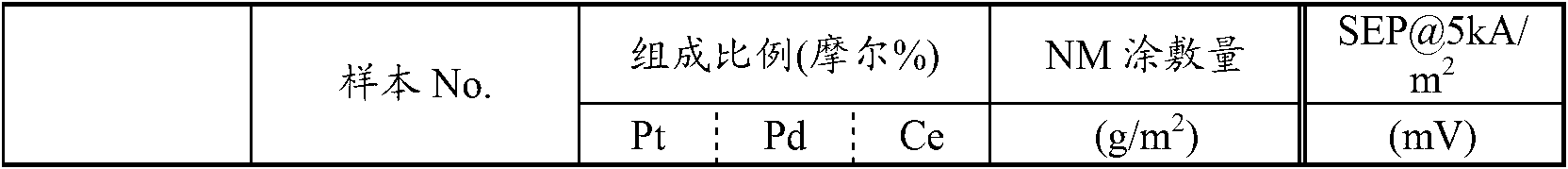
[0113] [table 3]
[0114]
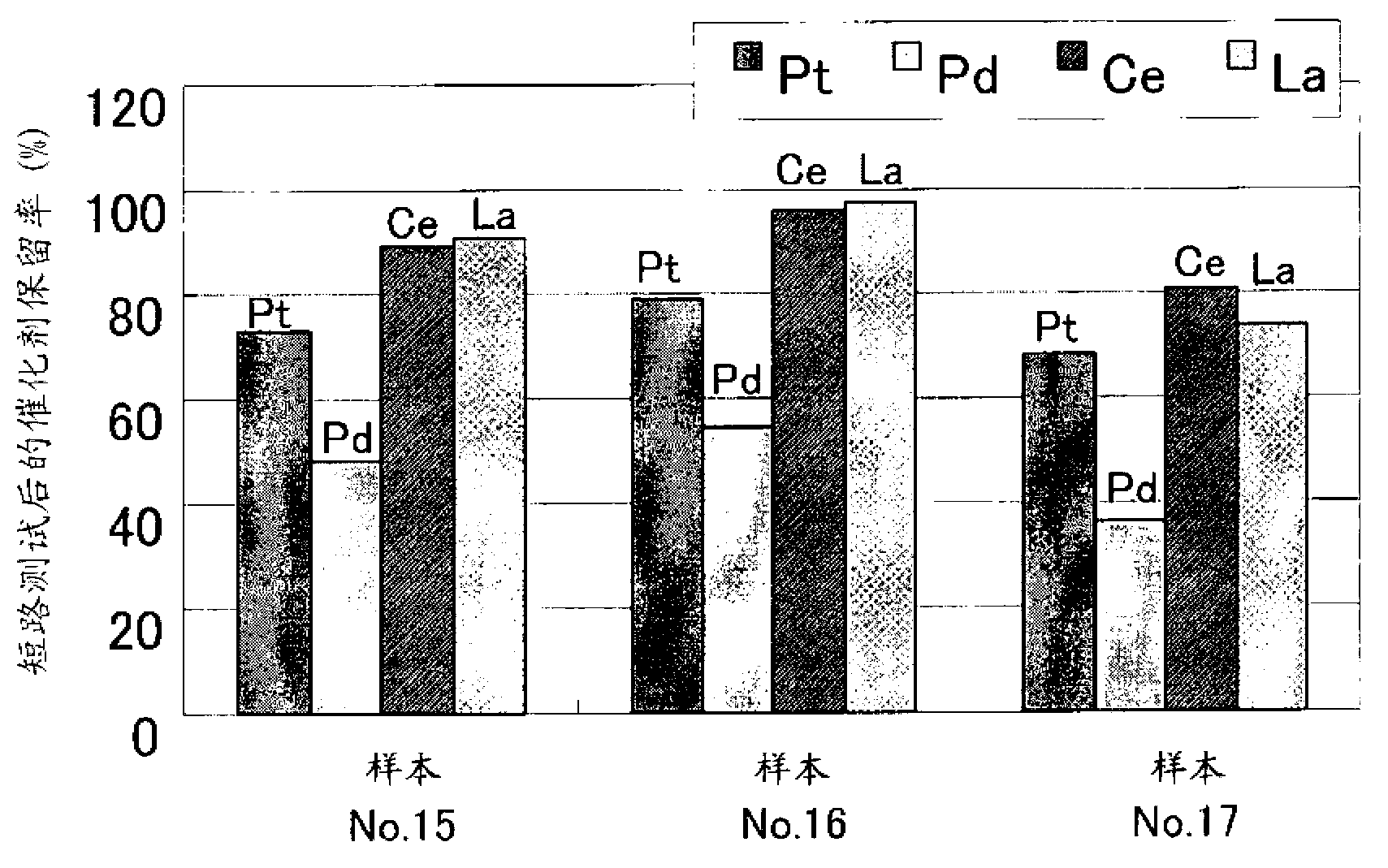
[0115] As shown in Table 4, the cathode samples No. 13 and No. 14 for hydrogen production showed good results in catalyst element retention after the short-circuit test and high current density test. In particular, the retention of platinum (Pt), which is the most important element for keeping the hydrogen overvoltage low, was very high in both samples, and even with a short circuit and operation at high current densities, it was possible to obtain Long service life with low hydrogen overvoltage.
[0116] [Table 4...
PUM
| Property | Measurement | Unit |
|---|---|---|
| porosity | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| current density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com