Flupirtine maleate sustained release tablet
A flupirtine acid sustained-release tablet, flupirtine maleate technology, applied in the direction of nervous system diseases, active ingredients of heterocyclic compounds, drug combination, etc., can solve side effects, large fluctuations in blood drug concentration, Malay Short half-life of flupirtine and other problems to achieve the effect of relieving pain
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
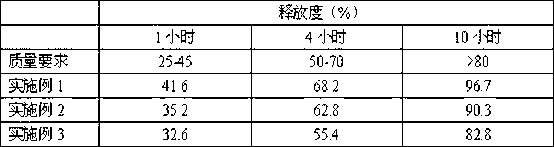
Embodiment 1
[0028] Example 1: A flupirtine maleate sustained-release tablet, consisting of the following components (the coating weight of the sustained-release granules increases by 1%):
[0029] Flupirtine maleate immediate-release granules: (based on the amount of flupirtine maleate) 100g
[0030] Flupirtine maleate sustained-release granules: (based on the amount of flupirtine maleate) 300g
[0031] Sodium carboxymethyl starch 30g
[0032] Micronized silica gel 25g
[0034] Microcrystalline Cellulose 20g
[0035] Among them, flupirtine maleate immediate-release granules consist of the following components:
[0036] Flupirtine Maleate 100g
[0037] Hypromellose (E50) 1g
[0038] Flupirtine maleate sustained-release granules are composed of the following components:
[0039] Flupirtine Maleate 300g
[0040] Hypromellose (E50) 9g
[0041] Microcrystalline Cellulose 30g
[0042] Eudragit NE30D 15ml
[0043] Calcium hydrogen phosphate 0.6g
[0044...
Embodiment 2
[0053] Example 2: A flupirtine maleate sustained-release tablet, consisting of the following components (the coating weight of sustained-release granules increased by 2%):
[0054] Flupirtine maleate immediate-release granules: (based on the amount of flupirtine maleate) 100g
[0055] Flupirtine maleate sustained-release granules: (according to the amount of flupirtine maleate) 300g
[0056] Sodium carboxymethyl starch 30g
[0057] Micronized silica gel 25g
[0059]Microcrystalline Cellulose 20g
[0060] Among them, flupirtine maleate immediate-release granules consist of the following components:
[0061] Flupirtine Maleate 100g
[0062] Hypromellose (E50) 1g
[0063] Flupirtine maleate sustained-release granules are composed of the following components:
[0064] Flupirtine Maleate 300g
[0065] Hypromellose (E50) 9g
[0066] Microcrystalline Cellulose 30g
[0067] Eudragit NE30D 30ml
[0068] Calcium hydrogen phosphate 1.2g
[0069]...
Embodiment 3
[0071] Example 3: A flupirtine maleate sustained-release tablet, consisting of the following components (the coating weight of the sustained-release granules increases by 3%):
[0072] Flupirtine maleate immediate-release granules: (based on the amount of flupirtine maleate) 100g
[0073] Flupirtine maleate sustained-release granules: (according to the amount of flupirtine maleate) 300g
[0074] Sodium carboxymethyl starch 30g
[0075] Micronized silica gel 25g
[0077] Microcrystalline Cellulose 20g
[0078] Among them, flupirtine maleate immediate-release granules consist of the following components:
[0079] Flupirtine Maleate 100g
[0080] Hypromellose (E50) 1g
[0081] Flupirtine maleate sustained-release granules are composed of the following components:
[0082] Flupirtine Maleate 300g
[0083] Hypromellose (E50) 9g
[0084] Microcrystalline Cellulose 30g
[0085] Eudragit NE30D 45ml
[0086] Calcium hydrogen phosphate 1.8g
[...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com