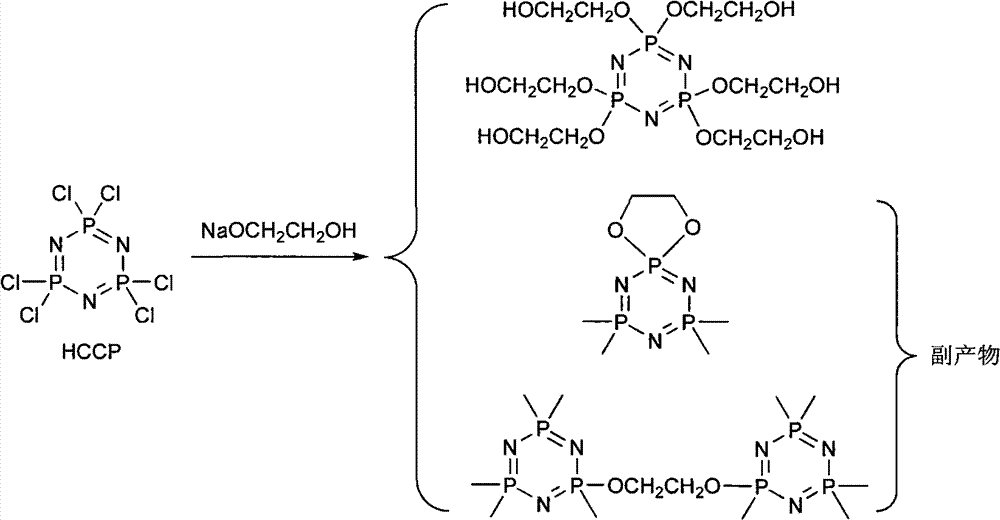
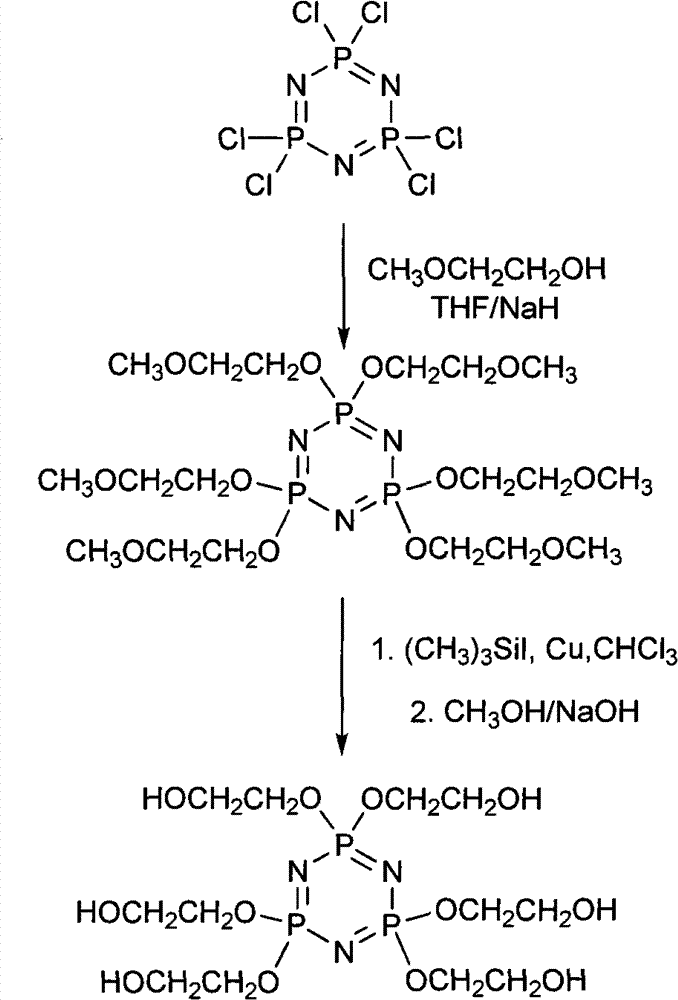
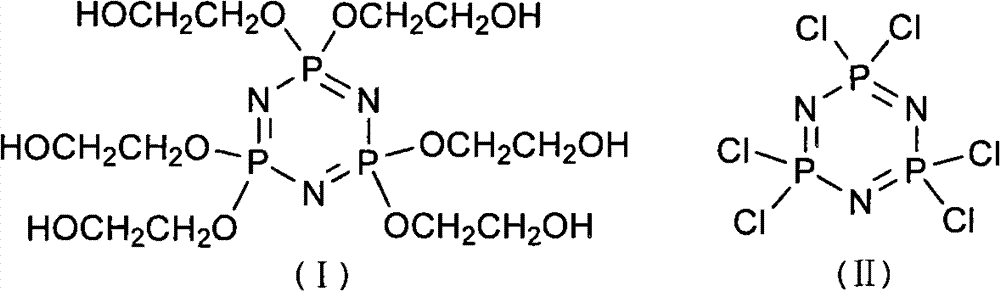
Synthetic method of 6(4-hydroxyl ethyoxyl) cyclotriphophazene
A technology of hydroxyethoxy and hexachlorocyclotriphosphazene, which is applied in chemical instruments and methods, compounds of Group 5/15 elements of the periodic table, organic chemistry, etc., can solve problems such as low yield, and achieve the reaction yield. High-rate, simple post-processing effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] (1) Synthesis of six (4-methoxyethoxy) cyclotriphosphazene
[0025] Add 300ml of tetrahydrofuran and 149.28g of sodium hydride (50%?) into a 1L three-necked reaction flask, then add 50ml of tetrahydrofuran solution containing 157.78g of methoxyethanol dropwise to the reaction flask under stirring at a temperature of 20°C to 30°C, After dropping, react at a temperature of 30° C. for 30 minutes. Then the temperature was lowered to 5°C, and 100ml of a tetrahydrofuran solution dissolved with 100g of hexachlorocyclotriphosphazene was added dropwise to the reaction flask, and reacted at a temperature of 50°C for 30h after the drop was completed. Filter the reaction solution, and evaporate the tetrahydrofuran in the obtained filtrate under reduced pressure to obtain a light yellow turbid viscous liquid, then add 150ml of dichloromethane to the liquid, wash the above liquid with distilled water until the water phase is neutral, separate the organic phase, reduce Dichloromethan...
Embodiment 2
[0046] (1) Synthesis of six (4-methoxyethoxy) cyclotriphosphazene
[0047] Add 300ml of tetrahydrofuran and 129.60g of sodium hydride (50%) into a 1L three-necked reaction flask, then add dropwise 50ml of tetrahydrofuran solution containing 164.35g of methoxyethanol to the reaction flask under stirring at a temperature of 20°C to 30°C, dropwise After completion, react at a temperature of 30° C. for 45 minutes. Then the temperature was lowered to 5°C, and 100ml of a tetrahydrofuran solution dissolved with 100g of hexachlorocyclotriphosphazene was added dropwise to the reaction flask, and reacted at a temperature of 50°C for 30h after the dropping was completed. After the reaction was completed, filter the reaction solution, distill off the tetrahydrofuran in the resulting filtrate under reduced pressure to obtain a light yellow turbid viscous liquid, then add 150ml of dichloromethane to the liquid, wash the above liquid with distilled water until the water phase is neutral, sep...
Embodiment 3
[0051] (1) Synthesis of six (4-methoxyethoxy) cyclotriphosphazene
[0052] Add 300ml of tetrahydrofuran and 129.60g of sodium hydride (50%) into a 1L three-necked reaction flask, then add dropwise 50ml of tetrahydrofuran solution containing 164.35g of methoxyethanol to the reaction flask under stirring at a temperature of 20°C to 30°C, dropwise After completion, react at a temperature of 35° C. for 40 minutes. Then the temperature was lowered to 0°C, and 100ml of tetrahydrofuran solution in which 100g of hexachlorocyclotriphosphazene was dissolved was added dropwise to the reaction flask, and reacted at 60°C for 24 hours after dropping. After the reaction was completed, filter the reaction solution, distill off the tetrahydrofuran in the resulting filtrate under reduced pressure to obtain a light yellow turbid viscous liquid, then add 150ml of dichloromethane to the liquid, wash the above liquid with distilled water until the water phase is neutral, separate the organic phase...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com