Preparation method of 2-(chloromethyl)-5,6-dimethoxy-3-methyl-1,4-para benzoquinone
A technology of trimethoxytoluene and dimethoxy, which is applied in the preparation of quinone oxide, organic chemistry, etc., can solve the problems of limited industrial application value, harsh reaction conditions, and difficulty in obtaining it. The method is simple and practical, the reaction operation is simple, low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
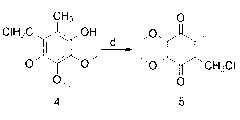
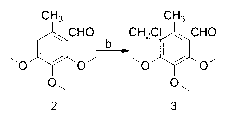
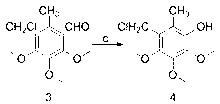
[0028] Example 1: The preparation method of 2-chloromethyl-5,6-dimethoxy-3-methyl-1,4-p-benzoquinone, the specific operation is as follows:
[0029] (1) Weigh 9.1g (0.05mol) of 3,4,5-trimethoxytoluene and dissolve it in 6.5ml N,N-dimethylformamide (0.09mol). Under nitrogen protection, control the reaction temperature at 0°C. Then 9.2g (0.06mol) of phosphorus oxychloride was slowly added dropwise, and the drop was completed after 1 hour, then the temperature was raised to 60°C for 10 hours, and the reaction was stopped. The reaction solution was poured into 200g of crushed ice and stirred for reaction. The mass percentage concentration is 30% sodium hydroxide solution to neutralize to neutral, a large amount of solids are precipitated, and compound 2 is obtained by filtration, which is a light yellow solid, melting point: 59-60 ° C, yield: 97%. 1 HNMR (500MHz, CDCl 3 ): 10.28 (s, 1H, -CHO), 3.86-3.73 (t, 9H, -OCH 3 ), 2.44(s,3H, -CH 3 );
[0030] (2) Weigh 3.2g (0.015mol) o...
Embodiment 2
[0034] Example 2: The preparation method of 2-chloromethyl-5,6-dimethoxy-3-methyl-1,4-p-benzoquinone, the specific operation is as follows:
[0035] (1) Weigh 9.1g (0.05mol) of 3,4,5-trimethoxybenzene and dissolve it in 4.5ml N,N-dimethylformamide (0.06mol). Under the protection of nitrogen, control the reaction temperature at 5°C and slowly Add 9.20 g (0.06 mol) of phosphorus oxychloride dropwise, and drop it for about 1.5 hours, then raise the temperature to 80°C and react for 7 hours, stop the reaction, pour the reaction solution into 150 g of crushed ice and stir the reaction, and finally the solution is mixed with 30% The sodium hydroxide solution was neutralized to neutral, a large amount of solids were precipitated, and compound 2 was obtained by filtration as a light yellow solid; melting point: 59-60°C, yield: 98%. 1 HNMR (500MHz, CDCl 3 ): 10.28 (s, 1H, -CHO), 3.86-3.73 (t, 9H, -OCH 3 ), 2.44(s,3H, -CH 3 );
[0036] (2) Weigh 3.2g (0.015mol) of compound 2 and 1.2...
Embodiment 3
[0040] Example 3: The preparation method of 2-chloromethyl-5,6-dimethoxy-3-methyl-1,4-p-benzoquinone, the specific operation is as follows:
[0041] (1) Weigh 9.1g (0.05mol) of 3,4,5-trimethoxybenzene and dissolve it in 7.5ml N,N-dimethylformamide (0.1mol). Under nitrogen protection, control the reaction temperature at 3°C , then slowly drop phosphorus oxychloride 15.2g (0.10mol), drop after 2 hours, then heat up to 100 ° C for 4 hours, stop the reaction, pour the reaction solution into 300g of crushed ice and stir the reaction, and finally the solution Neutralize to neutral with 30% sodium hydroxide solution, a large amount of solids are precipitated, and compound 2 is obtained by filtration as a light yellow solid, melting point: 59-60°C, yield: 95%. 1 HNMR (500MHz, CDCl 3 ): 10.28 (s, 1H, -CHO), 3.86-3.73 (t, 9H, -OCH 3 ), 2.44(s,3H, -CH 3 );
[0042] (2) Weigh 3.2g (0.015mol) of compound 2 and 2.25g (0.075mol) of paraformaldehyde, mix them, and add dropwise 27.0ml of h...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com