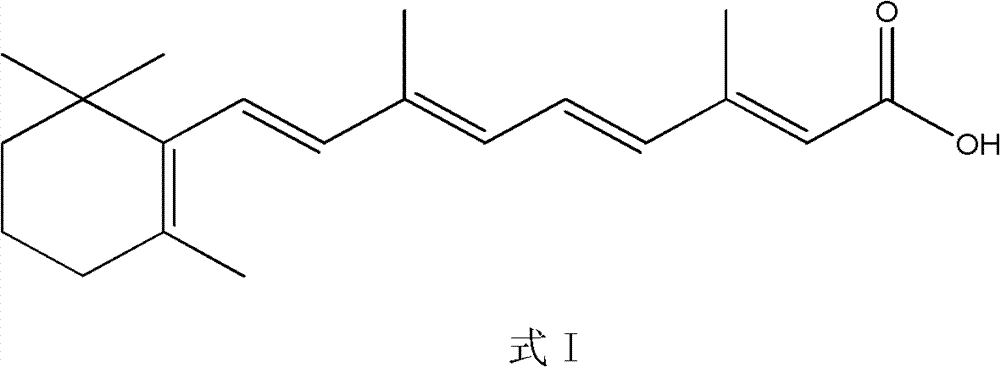
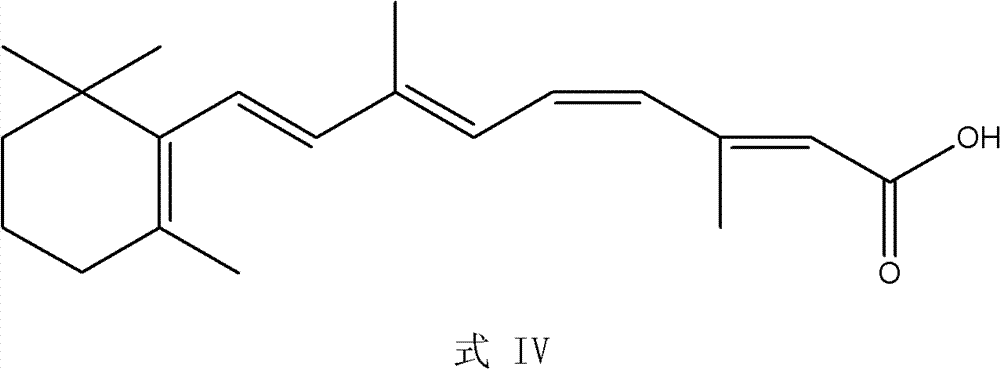
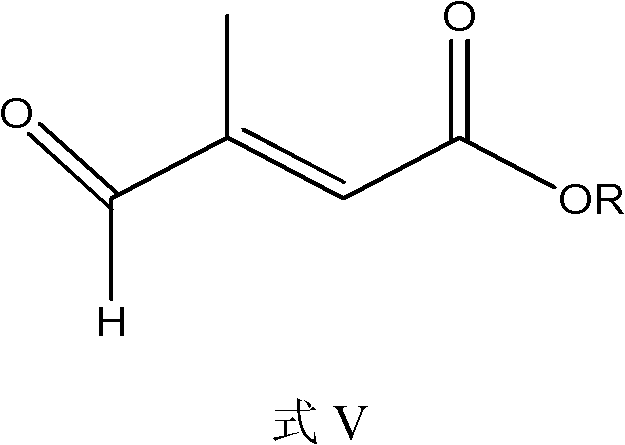
Synthesis method of all-trans-retinoic acid
A technology of all-trans and formyl crotonic acid, which is applied in the field of synthesis of all-trans retinoic acid, can solve the problems of incomplete time-consuming, multiple working hours, and high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 13
[0042] Example 13, the preparation of 7-dimethyl-9-(2,6,6-trimethylcyclohexen-1-yl)-2,4,6,8 all-trans nonatraenoic acid
[0043] 186g of [3-methyl-5-(2,6,6-trimethylcyclohexen-1-yl)-2,4-pentadiene]-triphenylphosphine chloride was dissolved in 500ml of isopropanol Add 42.3g of β-formyl crotonic acid under nitrogen gas, stir until the solution is clear, cool down to -5°C, add dropwise 571ml of 2N KOH isopropanol solution, keep the temperature at -5°C, and react for 2 hours. Then adjust the pH value to 7-8 with hydrochloric acid, add 86 mg palladium acetate, heat up to 50 ° C, and detect the reaction result by high performance liquid chromatography (HPLC). After the isomerization reaction is completed, pour it into water, add concentrated hydrochloric acid to neutralize, The crude product was obtained by suction filtration, and 95.2 g of the product was obtained by crystallization with ethyl acetate, with a purity of 99.7% and a yield of 85.3%.
Embodiment 2、3 and comparative test example 1、2
[0044] Embodiment 2, 3 and comparative test example 1, 2 - different isomerization pH conditions
[0045] Change the pH value regulated by hydrochloric acid, and others are carried out according to the conditions and methods of Example 1, and the obtained results are as follows:
[0046]
[0047] Conclusion: The pH value of the isomerization reaction is 5-10, the product has good purity and high yield.
Embodiment 4~7
[0048] Embodiment 4~7——different isomerization catalysts
[0049] Carry out according to the condition of embodiment 1, just change the kind of catalyst, obtained result is as follows:
[0050] Example
[0051] Conclusion: Several isomerization catalysts have good effect, and the obtained product has good purity and high yield.
[0052] Embodiment 8-10 and comparative test example 4——different isomerization temperature
[0053] Carry out according to the condition of embodiment 1, just change the temperature of isomerization reaction, gained result is as following table:
[0054]
[0055] Conclusion: The suitable temperature for isomerization reaction is 50~70℃.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com