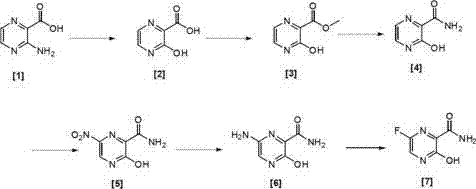
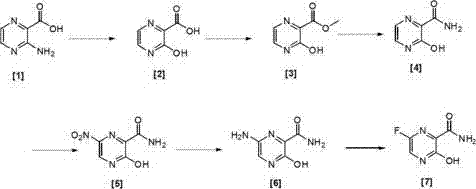
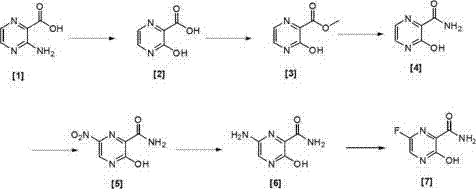
Preparation method of 6-fluoro-3-hydroxy-2-pyrazinamide
The technology of pyrazinamide and hydroxyl group is applied in the field of preparation of 6-fluoro-3-hydroxy-2-pyrazinamide, which can solve the problems of low yield of multi-step reaction, complicated operation steps, harsh reaction conditions, etc. The effect of easy control of conditions, high yield and mild process conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] CDCl 3 : deuterated chloroform
Embodiment 1-1
[0075]
[0076] Under nitrogen protection, 14g (100.7mmol, 1.0eq) of [1] was dissolved in 300ml of 1M hydrochloric acid, and 8.3g (200.8mmol, 1.2eq) of NaNO was added under stirring. 2 , stirred in an ice bath for 3 h, TLC detected that the reaction was complete, and the reaction was stopped. Then the reaction solution was poured into 5 times the volume of water to precipitate a solid, filtered, and the filter cake was vacuum-dried at room temperature to obtain 12.7 g of [2] with a yield of 90%. 1 H-NMR (CDCl 3 ,400MHz) δ value: 8.50(1H,d,pyrazine H,J=12Hz),8.53(1H,d,pyrazine H,J=12Hz),11.45(1H,s,OH),11.53(1H,s,COOH ).
Embodiment 1-2
[0078]
[0079] Under the protection of nitrogen, 12.7g (90.7mmol) of [2] was dissolved in 150ml of anhydrous methanol, and concentrated sulfuric acid (15ml) was added dropwise with stirring, and the reaction system was stirred at 40°C for 5h. TLC detected that the reaction was complete and stopped. reaction. Finally, 8 times the volume of water / ethyl acetate (5 / 3) was added, stirred for 5 min, separated, the organic phase was washed with saturated brine, dried over anhydrous sodium sulfate, and rotary evaporated to obtain 12.8 g of [3]. The yield was 92%. 1 H-NMR (CDCl 3 ,400MHz) δ value: 4.09 (3H,s,CH 3 ), 8.50 (1H, d, pyrazine H, J=12Hz), 8.53 (1H, d, pyrazine H, J=12Hz), 11.44 (1H, s, OH).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com