Asymmetric cationic Gemini surfactant and preparation method thereof
A surfactant and cation technology, applied in chemical instruments and methods, preparation of amino-substituted functional groups, organic chemistry, etc., to reduce the difficulty and improve the reaction yield.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
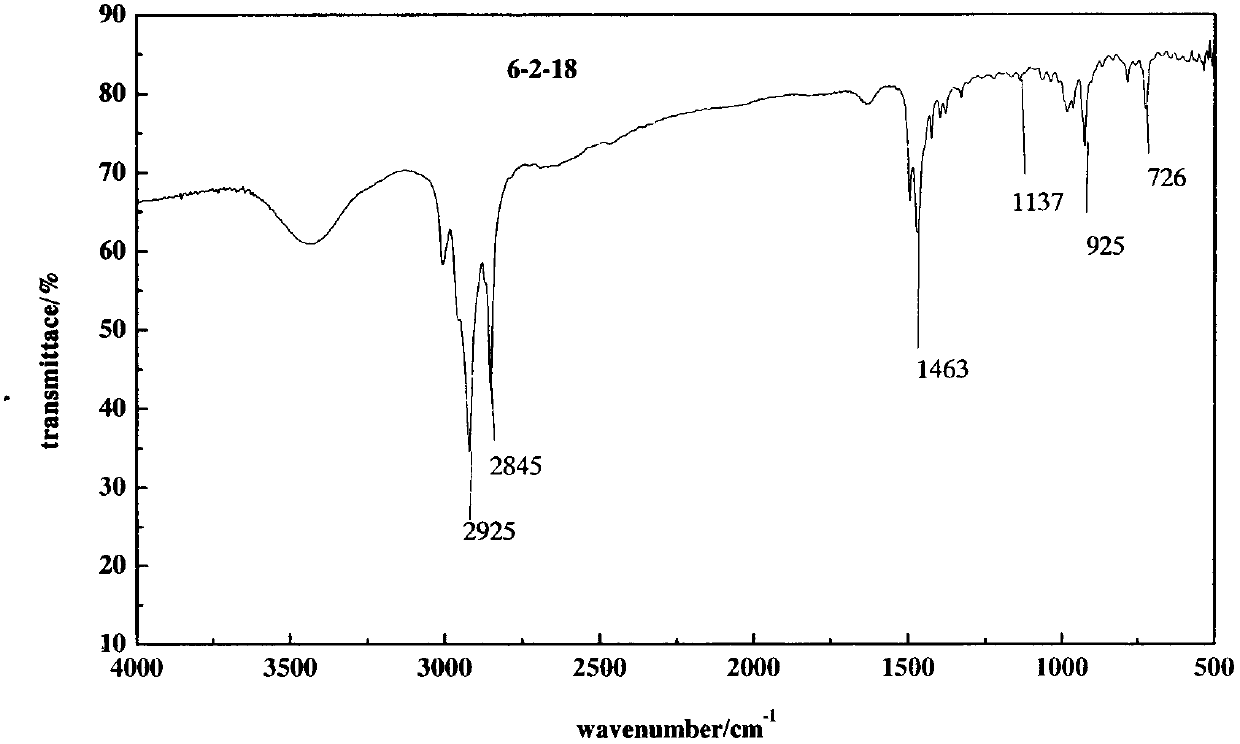
[0048] Synthesis of 6-2-18
[0049] (1) synthesis
[0050] In a 250mL round bottom flask, add ethylenediamine and bromo-n-hexane according to the molar ratio of ethylenediamine and bromo-n-hexane in the amount of 5:1, and then add 100mL of absolute ethanol. Magnetic stirring, react at 50°C for 24 hours, then raise the temperature to the reflux temperature of ethanol (80-85°C) and continue to react for 48 hours, cool down, spin the ethanol to dryness, add about 50mL of petroleum ether to the crude product, stir, and let stand to separate layer, the upper layer liquid was poured off, and this operation was repeated three times to obtain a light yellow solid.
[0051] Add octadecyl bromide in a molar amount of 1.2 times the amount of the original n-bromohexane to the washed product, add 100 mL of absolute ethanol, and heat up to 80-85° C. to continue the reaction for 72 hours.
[0052] (2) Purification
[0053] Heat and dissolve the crude product with about 10 mL of absolute e...
Embodiment 2
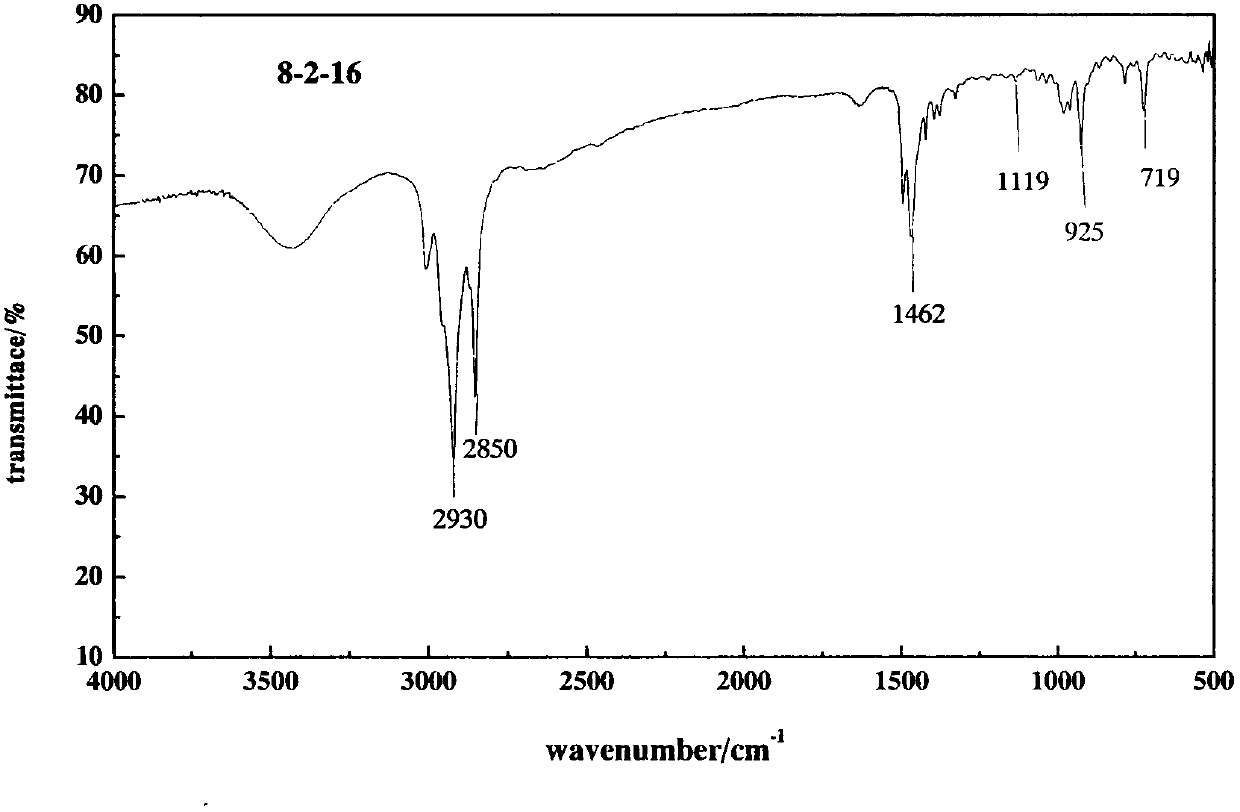
[0055] Synthesis of 8-2-16
[0056] (1) synthesis
[0057] In a 250mL round bottom flask, add ethylenediamine, n-octane bromide and 100mL absolute ethanol according to the molar ratio of ethylenediamine and n-octane bromide in a molar ratio of 5:1. Stir magnetically and react at 50°C for 24 hours. Then heat up to the reflux temperature of ethanol (80-85° C.) to continue the reaction for 48 hours, cool down, spin the ethanol to dryness, add about 50 mL of petroleum ether to the crude product, stir, let stand to separate layers, pour off the upper liquid, and repeat this operation three times Repeatedly, a light yellow solid was obtained.
[0058] Add hexadecane bromide in a molar amount that is 1.2 times the amount of the original n-octane bromide to the washed product, add 100 mL of absolute ethanol, and heat up to 80-85° C. to continue the reaction for 72 hours.
[0059] (2) Purification
[0060] Heat and dissolve the crude product with about 10mL of absolute ethanol, and ...
Embodiment 3
[0062]Determination of Surface Tension of Asymmetric Cationic Gemini Surfactant
[0063] Adopt ring method to measure the surface tension of product solution under different concentrations, make 6-2-18 and 8-2-16 aqueous solution surface tension change curve with concentration, see Figure 5 and Figure 6 . The critical micelle concentration value (cmc) and the surface tension γ at the critical micelle concentration are obtained from the turning point of the curve in the figure cmc . Experiments have found that the critical micelle concentration and surface tension at the critical micelle concentration of asymmetric cationic Gemini surfactants are low, and the cmc of 6-2-18 and 8-2-16 is not much different at 6mmol / L. Gamma of surface tension 6-2-18 at micellar concentrations cmc 41.9mN·m -1 , 8-2-16's cmc 34.9mN·m -1 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com