Method for preparing 3-chloro-4-methylaniline through catalytic hydrogenation
A technology for catalytic hydrogenation and methylaniline, which is applied in the preparation of amino compounds, chemical instruments and methods, preparation of organic compounds, etc., can solve the problems of reduction and high catalyst cost, and achieves simple process, high selectivity and good product quality. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
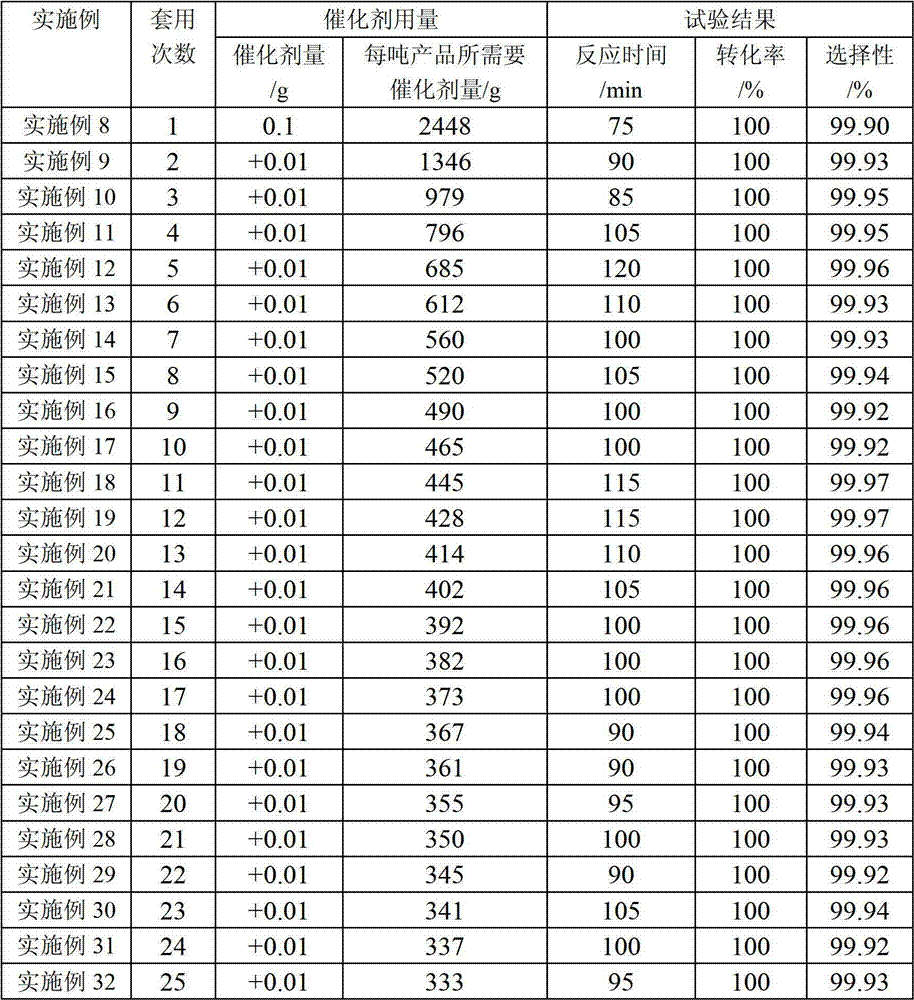
Examples
Embodiment 1
[0023] In a 500ml stainless steel high-pressure reactor, add 40.0g 3-chloro-4-methylnitrobenzene, 0.12g Pd-Fe / C catalyst (Pd content 5%, Fe content 0.8%), 120ml 95% ethanol-water solution (mass content ), close the reactor, replace the air with nitrogen 3 times, 0.2MPa each time, and replace the nitrogen with hydrogen 3 times, 0.2MPa each time. Add hydrogen to 2.0MPa, then raise the temperature to 65°C, start stirring, the hydrogen in the reactor drops to 1.5MPa, then add hydrogen to 2.0MPa, wait until the reading of the hydrogen pressure gauge no longer drops, the reaction is complete, cool down to room temperature, and use Nitrogen replaces the hydrogen in the autoclave, opens the reactor, takes out the reaction solution, filters out the catalyzer, and the filtrate distills off the solvent, obtains the 32.7g3-chloro-4-methylaniline product, analyzes its content with gas chromatography, and the results show that 3 -Chloro-4-methylaniline selectivity 99.92%, 3-chloro-4-methyla...
Embodiment 2
[0025] Except using 120ml 90% ethanol-water solution as solvent, other steps are identical with embodiment 1, obtain 32.7g3-chloro-4-methylaniline product, the content of each group of gas chromatography analysis product, the result shows, 3-chloro-4 -Methylaniline selectivity 99.90%, 3-chloro-4-methylaniline yield 99.0%.
Embodiment 3
[0027] Except using 120ml dehydrated alcohol as solvent, other steps are identical with embodiment 1, obtain 32.7g3-chloro-4-methylaniline product, the content of each group of product of gas chromatography analysis, the result shows, 3-chloro-4-methylaniline The selectivity of aniline is 99.95%, and the yield of 3-chloro-4-methylaniline is 99.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com