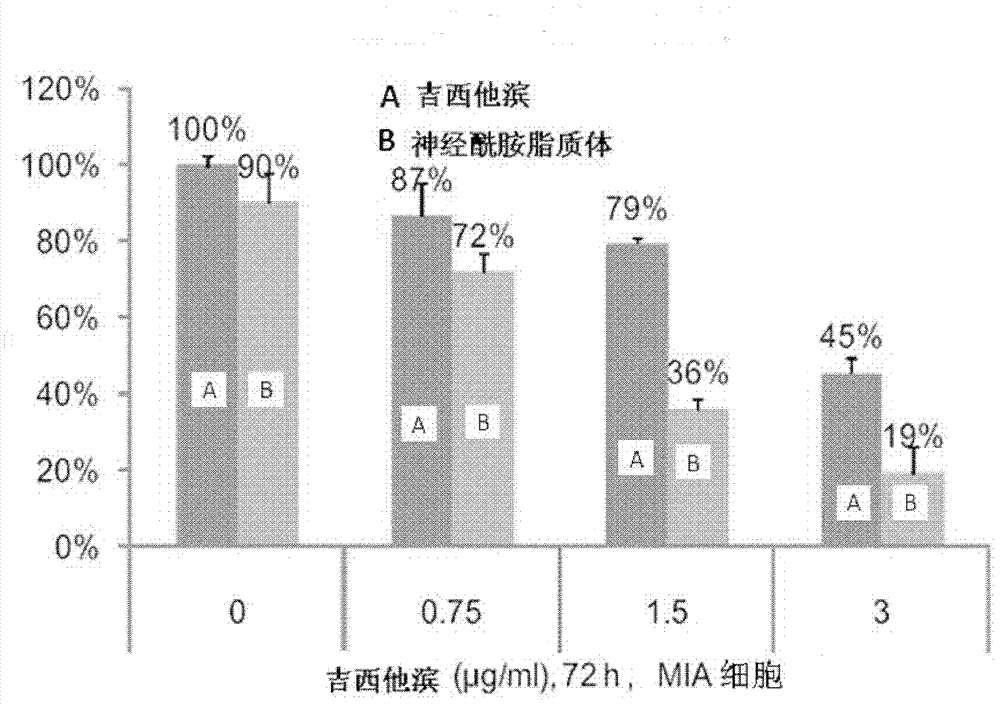
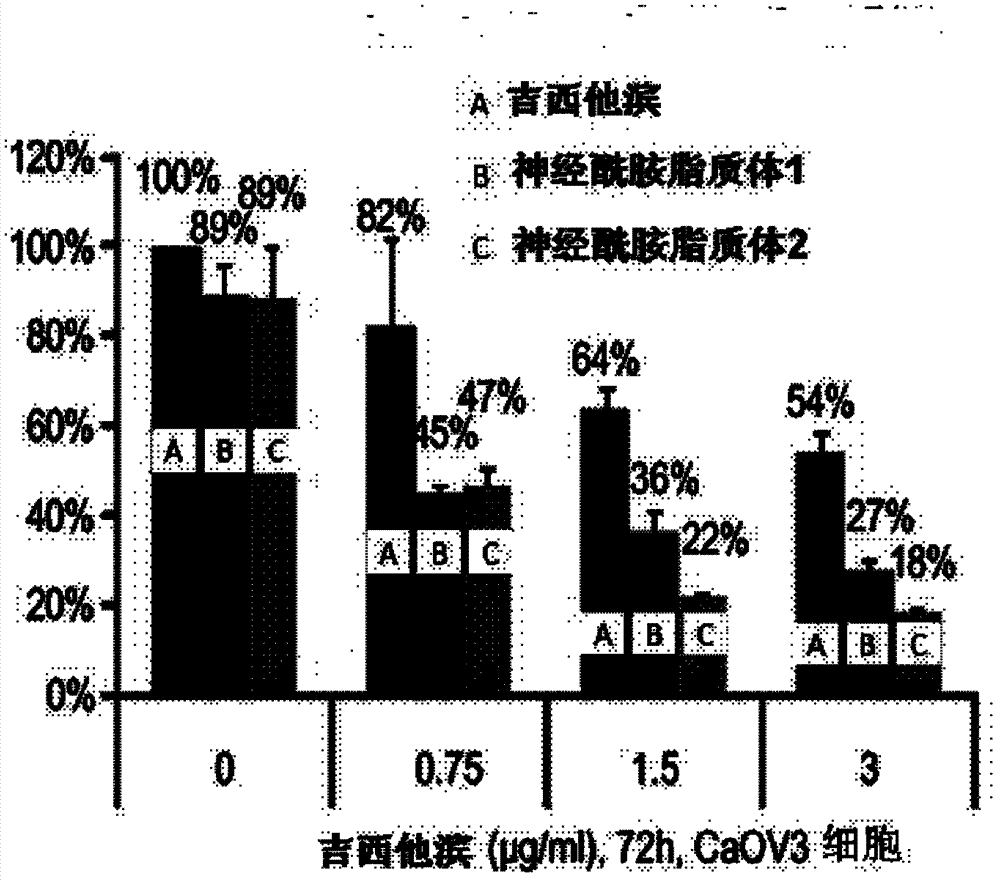
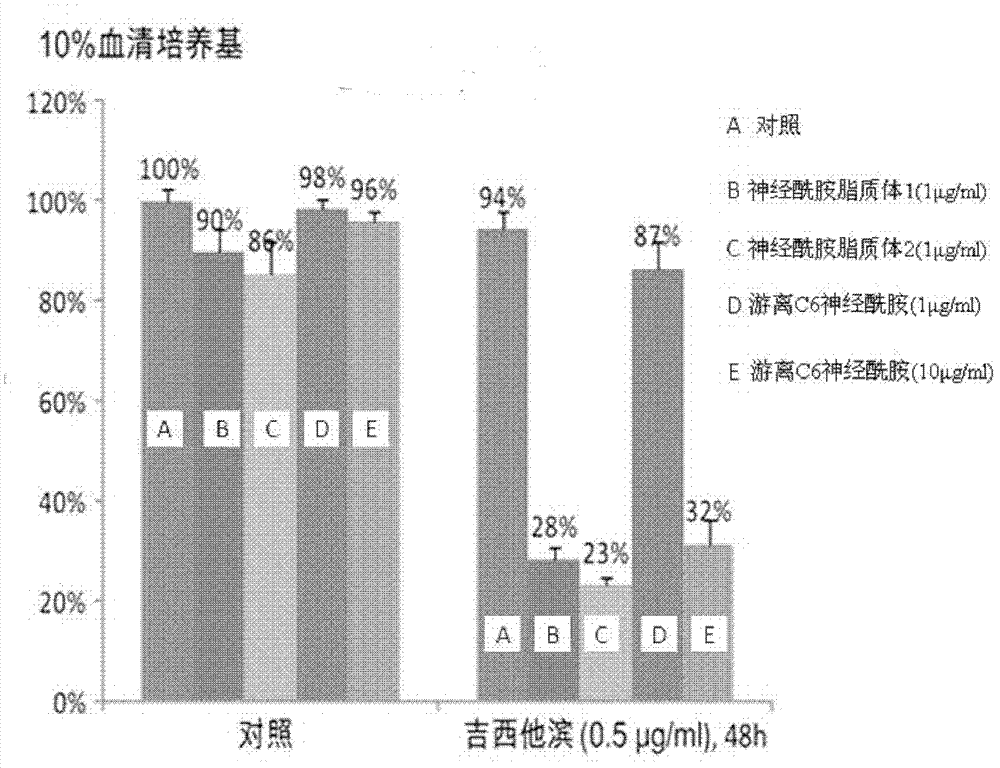
Ceramide liposome and preparation method and application thereof
A technology of ceramide lipids and ceramides, which is applied in the field of pharmaceutical preparations to achieve the effects of reducing the concentration of chemotherapy drugs, prolonging the survival time of organisms, and improving targeting and bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Ceramide 20mg
[0044] Cholesterol 200mg
[0045] Ether 10ml
[0046] Taurocholate 30mg
[0047] Preparation method: Dissolve 30mg taurocholate in phosphate buffer (pH 7.4), the weight concentration of taurocholate in phosphate buffer is 15%; mix 20mg ceramide, 20mg egg yolk lecithin and Dissolve 200mg of cholesterol in ether, then slowly inject this solution into phosphate buffer at 50-60℃, stir with an electronic constant speed stirrer at 600r / min until the ether is completely removed, and then dilute with the above phosphate buffer To 100ml, the ceramide liposome is obtained and stored at 4°C.
Embodiment 2
[0049] Ceramide 30mg
[0050] Soy Lecithin 120mg
[0051] Cholesterol 250mg
[0052] Methanol 10ml
[0053] Ethanol 10ml
[0054] Sodium deoxycholate 40mg
[0055] Preparation method: Dissolve 40mg of sodium deoxycholate in an appropriate amount of Tris-HCl (pH7.5, containing 0.15mol / L NaCl) buffer with a molar concentration of 20mmol / L. Sodium deoxycholate in Tris-HCl buffer The weight concentration is 10%; 30mg ceramide is dissolved in 10ml methanol, 120mg lecithin, 250mg cholesterol is dissolved in 10ml ethanol, the two solutions are put together in a rotary evaporator, and the solvent is removed under reduced pressure at 40℃ to form Film, then add the above, and then add the above Tris-HCl buffer dissolved with sodium deoxycholate, ultrasonically treat for 30 minutes, to obtain ceramide liposomes, and spray-dry to obtain a lyophilized preparation of ceramide liposomes . The lyophilized preparation only needs to be fully hydrated and shaken for a short time before use to re-form t...
Embodiment 3
[0057] Ceramide 100mg
[0058] Soy Lecithin 1200mg
[0059] Hydrogenated saturated phospholipids 300mg
[0060] Cholesterol 100mg
[0061] Ganglioside 50mg
[0063] Vitamin E 50mg
[0064] Preparation method: ultrasonically dissolve 100mg ceramide, 1200mg soy lecithin, 300mg hydrogenated saturated phospholipid, 100mg cholesterol, 50mg ganglioside and 6.5mg vitamin E in 50ml absolute ethanol, and place this solution in a rotary evaporator at 40℃ Remove the solvent under reduced pressure to form a thin film, and then add 20mmol / L Tris-HCl (pH7.5, containing 0.15mol / L NaCl) buffer, ultrasonic treatment for 30min, homogenize 5-6 times with a high-pressure homogenizer, and then pass 0.22μm The microporous filter membrane is packed and sealed to obtain ceramide liposomes, and dried to obtain a lyophilized preparation of ceramide liposomes. The lyophilized preparation only needs to be fully hydrated and shaken for a short time before use, and liposomes can be f...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com