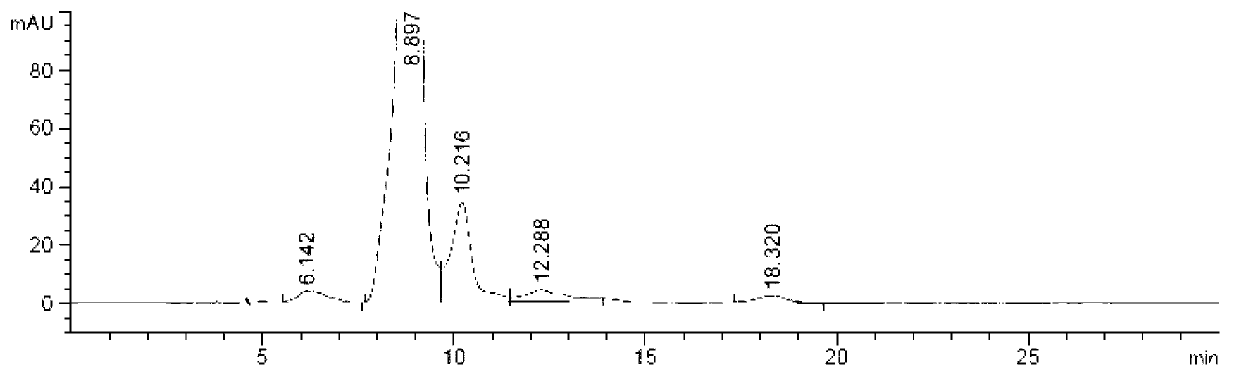
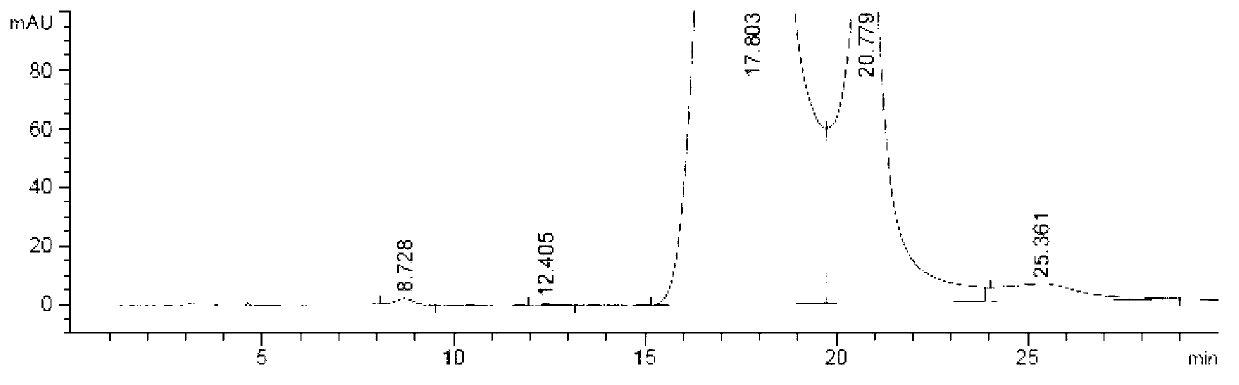
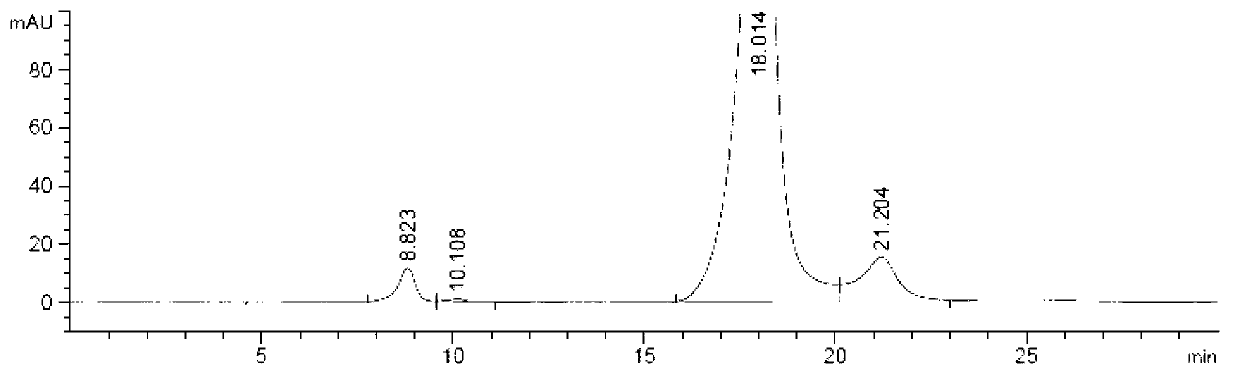
Preparation method of iohexol impurity
A technology of iohexol and compounds, applied in the field of medicinal chemistry, can solve the problems of low yield, shortage of reference substances, high price, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Example 1: Preparation of Formula (7) Compound 5-amino-3-N-(2,3-dihydroxypropyl)-2,4,6-triiodo-1,3-benzenedicarboxamide
[0048] Add formula (8) compound 3-amino-5-(2,3-diacetoxy n-propylcarbamoyl)-2,4,6-triiodobenzoic acid (100g) and 5 times the amount ( V / W) thionyl chloride (500ml), heated to reflux until dissolved, the reaction was completed, concentrated under reduced pressure to recover thionyl chloride, added 3 times the amount (V / W) of chloroform (300ml) into the reaction bottle, cooled to 10-20°C, add water to wash and separate the layers, separate the organic layer, concentrate to dryness under reduced pressure, add 3 times the amount (V / W) of THF (300ml), and pass through excess dry ammonia to make the pH of the reaction solution 10- 12. After the reaction is completed, add an appropriate amount of sodium hydroxide aqueous solution to adjust the pH=14. After hydrolysis at room temperature, cool to 10-20°C to precipitate crystals, filter, and dry to obtain the...
Embodiment 2
[0049] Example 2: Formula (5) compound 5-acetamido-3-N-(2,3-diacetoxypropyl)-2,4,6-triiodo-1,3-phthalamide preparation
[0050] Starting from the compound 5-amino-3-N-(2,3-dihydroxypropyl)-2,4,6-triiodo-1,3-benzenedicarboxamide of formula (7), the compound Add 30g (47.55mmol) into a 250ml reaction flask, add 120ml of acetic anhydride dropwise, add 0.1g of concentrated sulfuric acid dropwise, and raise the temperature to 65°C-70°C for 26 hours. TLC monitors the complete reaction of the raw materials. Concentrate under reduced pressure to dry to obtain sticky substance formula (5) compound 5-acetamido-3-N-(2,3-diacetoxypropyl)-2,4,6-triiodo-1,3-benzene Diformamide. Without purification, the next reaction was carried out directly.
Embodiment 3
[0051] Example 3: Preparation of Formula (6) Compound 5-acetamido-3-N-(2,3-dihydroxypropyl)-2,4,6-triiodo-1,3-phthalamide
[0052] Reduce the temperature of the viscous compound (5) prepared in Example 2 to 25°C-30°C, slowly add 20% sodium hydroxide solution dropwise under stirring until the reaction liquid is dissolved and adjust the pH value to 13-14, Insulate the reaction for 3 hours, and monitor the complete reaction of the raw materials in the liquid phase. Use concentrated hydrochloric acid to adjust the pH value to neutral, stir at room temperature for 12 hours until a large amount of white solids precipitate, filter the solids out and rinse with water three times, and dry to obtain the hydrolyzate of formula (6) compound 5-acetamido-3-N-(2 ,3-dihydroxypropyl)-2,4,6-triiodo-1,3-benzenedicarboxamide (22g, 62.5% / two-step yield).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com