Novel beta-aminopropanoic acid synthesis technology
A technology for aminopropionic acid and aminopropionic acid crude product, which is applied in the preparation of organic compounds, organic chemistry, chemical instruments and methods, etc., can solve the problems of β-aminopropionic acid destruction, unqualified finished product quality, long production time and the like, To achieve the effect of short concentration cycle, shortened recovery cycle and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
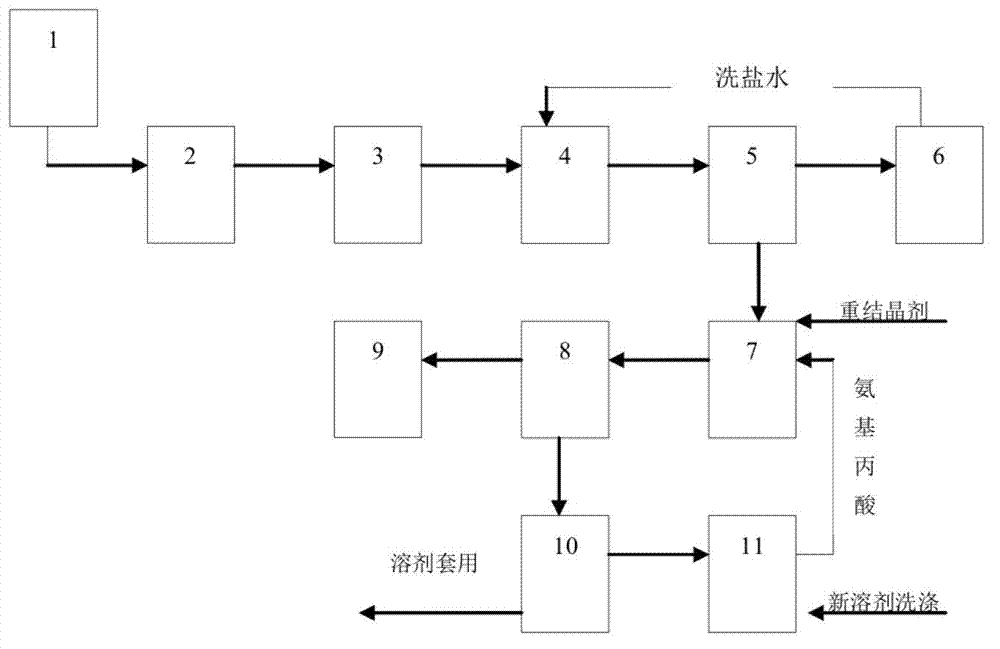
[0035] (1) Hydrolysis, temperature-controlled dropwise addition of β-aminopropionitrile in the β-aminopropionitrile metering tank 1 to the hydrolysis kettle 2 in which liquid caustic soda has been added to carry out the hydrolysis reaction;
[0036] (2) Neutralization, using hydrochloric acid to neutralize the hydrolyzed solution in the neutralization tank 3;
[0037] (3) Concentrate and concentrate in the concentration kettle 4 while maintaining a certain temperature and vacuum;
[0038] (4) Heat filtration, adding a salting-out agent and using a centrifuge 5 for direct heat filtration, and the filtrate is cooled and filtered to obtain the crude product of β-alanine;
[0039] (5) Synchronous operation, the filtrate obtained by filtering the crude product of β-alanine is returned to the concentrated and filtered cycle operation through the salt washing tank 6; at the same time, the crude product of β-alanine is dissolved to enter the next step;
[0040] (6) Recrystallization,...
Embodiment 2
[0044] hydrolysis:
[0045] 1. Add 211.2g of liquid caustic soda to the three-necked flask, raise the temperature to 80°C with a water bath, slowly open the 88g β-aminopropionitrile drop valve, and control the dropwise addition in 5-6 hours, the temperature is 80-90°C;
[0046] 2. At the end of the dropwise addition, keep warm for 30 minutes at a temperature of 90-95°C and a pressure of -0.02MPa;
[0047] 3. After the heat preservation is completed, the ammonia is driven by vacuum.
[0048] neutralize:
[0049] 1. Cool down to about 40°C and add 230g of hydrochloric acid dropwise, and the neutralization temperature is below 50°C;
[0050] 2. When adding dropwise until 10g of hydrochloric acid remains, check the pH, continue to add dropwise until the pH is 7, and retest the pH value after half an hour.
[0051] Concentration: Keep the decompression outlet water temperature at 85-100°C and the vacuum degree above -0.09MPa.
[0052] Hot Filtration: After adding salting-out ag...
Embodiment 3
[0063] hydrolysis:
[0064] 1. Add 105.6g of liquid caustic soda into the three-necked flask, raise the temperature to 80°C with a water bath, slowly open the 44g aminopropionitrile drop valve, control the dropwise addition within 2-3 hours, and the temperature is 80-90°C;
[0065] 2. At the end of the dropwise addition, keep warm for 30 minutes at a temperature of 90-95°C and a pressure of -0.02MPa;
[0066] 3. After the heat preservation is completed, the ammonia is driven by vacuum.
[0067] neutralize:
[0068] 1. Cool down to about 40°C and prepare to add 115g of hydrochloric acid dropwise, and the neutralization temperature is below 50°C;
[0069] 2. When adding dropwise until 10g of hydrochloric acid remains, check the pH, continue to add dropwise until the pH is 7, and retest the pH value after half an hour.
[0070] Concentration: Keep the decompression outlet water temperature at 85-100°C and the vacuum degree above -0.09MPa.
[0071] Hot Filtration: After adding...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com