Codon-optimized 7 beta-hydroxy steroid dehydrogenase gene
A hydroxysteroid and codon optimization technology, applied in the field of 7β-hydroxysteroid dehydrogenase (7β-HSDH) gene, can solve the problems of low yield and complicated chemical synthesis process, and achieve the effect of simple and fast purification method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0013] Example 1: Optimum Design and Synthesis of 7β-Hydroxysteroid Dehydrogenase Gene
[0014] Analysis of rare codons: rare codons are mainly analyzed by the software E.coli Codon Usage Analysis2.0 (Morris Maduro, reference: Analysis and Predictions from Escherichia coli sequences in: Escherichia coli and Salmonella, Vol.2, Ch.114:2047- 2066, 1996, Neidhardt FC ed., ASM press, Washington, D.C.) analyzed the codons whose usage frequency in Escherichia coli was lower than the threshold (10‰).
[0015] Optimize rare codons: optimize the codons whose usage frequency is lower than the threshold according to the usage frequency of different codons in E. coli. The optimization results are as follows:
[0016] Optimized codons used in the present invention
[0017] before optimization
Optimized
before optimization
Optimized
AAG
AAA
Lys
CTAs
CTG
Leu
ACA
ACC
Thr
CTT
CTG
Leu
...
Embodiment 2
[0020] Example 2: Construction of the recombinant expression vector pGEX-6p-1-7β-HSDH of the optimized 7β-hydroxysteroid dehydrogenase gene and the acquisition of Escherichia coli containing the recombinant expression vector
[0021] ⑴ strains and plasmids
[0022] Escherichia coli: BL21 strain, purchased from Tianjin Bomeike Biotechnology Co., Ltd.
[0023] Expression vector pGEX-6p-1: purchased from GE (GE Healthcare).
[0024] (2) Construction of recombinant expression vector and acquisition of Escherichia coli containing the recombinant expression vector
[0025] Use restriction endonuclease BamHI / NotI to double digest the optimized gene and recover the target gene fragment; use restriction endonuclease BamHI / NotI to double digest the prokaryotic expression vector pGEX-6p-1 and recover the target vector fragment; ligate, Escherichia coli was transformed by heat shock method; positive clones were screened by PCR, and then verified by sequencing to obtain recombinant expre...
Embodiment 3
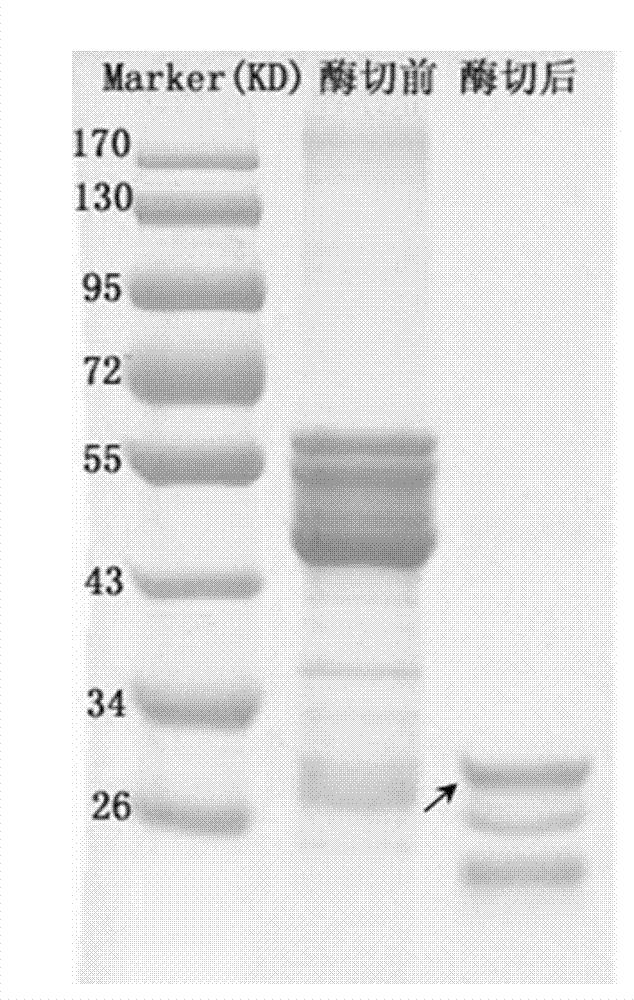
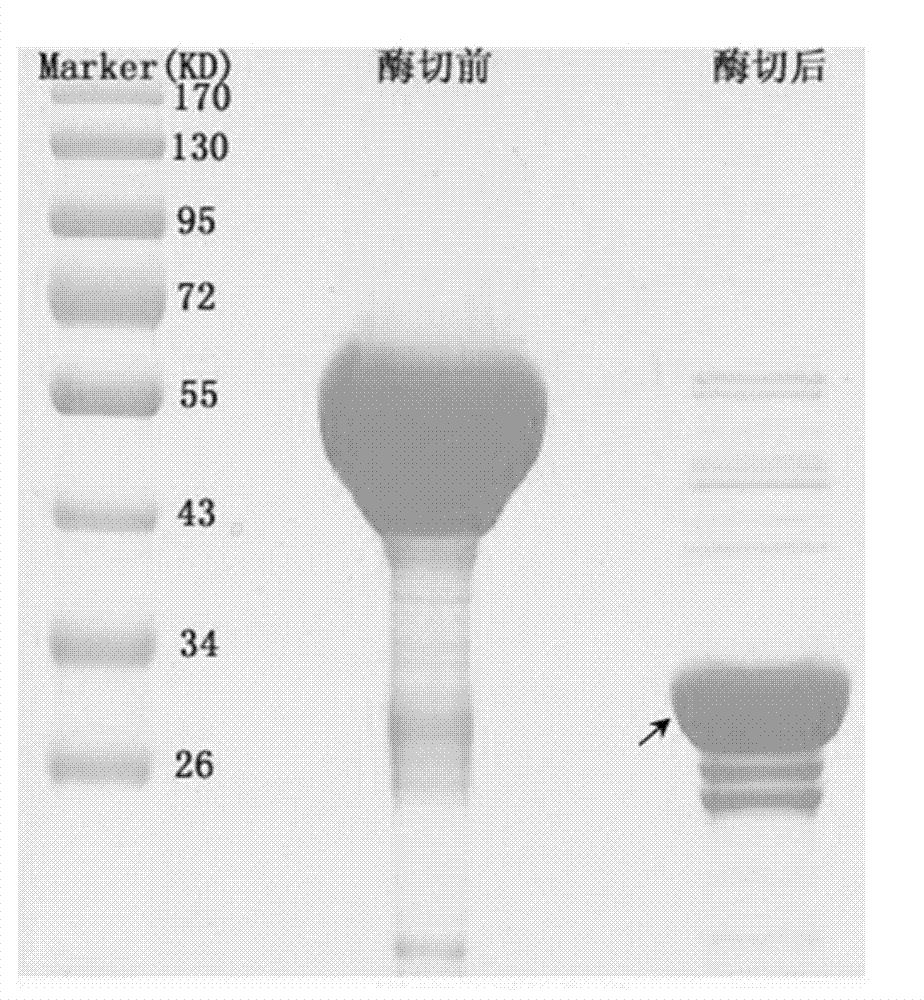
[0026] Example 3: Expression of optimized 7β-hydroxysteroid dehydrogenase gene in Escherichia coli
[0027] The successfully transferred E. coli recombinant strains were added to the LB liquid medium at an addition rate of 5%, and cultured at 220 rpm and 37°C. When the OD600 is 0.5, induce with IPTG at 16°C for 12 hours to make Escherichia coli produce GST fusion protein.
[0028] Centrifuge the induced Escherichia coli at 10,000rpm for 3min to collect the bacterial cells, and ultrasonically break the cell wall at 300W for 5min to clarify; centrifuge at 14,000rpm at 4°C for 15min to collect the supernatant; medium (purchased from GE healthcare company) for 2 hours at 4°C to bind the GST fusion protein to the glutathione agarose gel, and then wash three times with pre-cooled five-fold volume of 0.25% Tween 20 in PBS (100mM) , washed three times with pre-cooled five-fold volume of PBS (100mM). The glutathione agarose gel containing the GST fusion protein was directly digested ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com